Basic fibroblast growth factor stimulates matrix metalloproteinase-13 via the molecular cross-talk between the mitogen-activated protein kinases and protein kinase Cdelta pathways in human adult articular chondrocytes
- PMID: 17311929
- PMCID: PMC2895271
- DOI: 10.1074/jbc.M609040200
Basic fibroblast growth factor stimulates matrix metalloproteinase-13 via the molecular cross-talk between the mitogen-activated protein kinases and protein kinase Cdelta pathways in human adult articular chondrocytes
Abstract
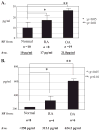
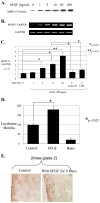
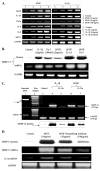
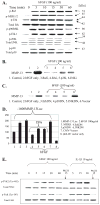
Excessive release of basic fibroblast growth factor (bFGF) during loading and/or injury of the cartilage matrix may contribute to the onset or progression of osteoarthritis. This pathological role may be related to the ability of bFGF to decrease proteoglycan synthesis and to antagonize the activity of anabolic growth factors in cartilage such as insulin-like growth factor-1 and bone morphogenetic protein 7 (BMP7 or OP-1). Matrix metalloproteinase-13 (MMP-13), a catabolic cartilage-degrading enzyme, is dramatically up-regulated by inflammatory cytokines or by fibronectin fragments in articular chondrocytes. In this study, we investigated MMP-13 production by bFGF using human articular chondrocytes. Endogenous concentration of bFGF in synovial fluids collected from arthritis patients and asymptomatic subjects showed a good linear correlation with the endogenous levels of MMP-13. bFGF stimulation of MMP-13 was mediated at the transcriptional level and, at least in part, by stimulation of interleukin-1 production. Also, our findings suggest that bFGF stimulation of MMP-13 required the activation of multiple MAPKs (ERK, p38, and JNK) by bFGF, and more importantly, bFGF activation of protein kinase C (PKC) delta played a key role in the MMP-13 stimulation. Indeed, PKCdelta is the only isoform associated with MMP-13 stimulation among the PKC isoforms tested. PKCdelta controls the bFGF response by regulating multiple MAPK pathways. Our results suggest that PKCdelta activation is a principal rate-limiting event in the bFGF-dependent stimulation of MMP-13 in human adult articular chondrocytes. We propose that deregulation of cross-talk between MAPK and PKCdelta signaling may contribute to the etiology of osteoarthritis in human patients.
Figures


Similar articles
-
Basic fibroblast growth factor activates the MAPK and NFkappaB pathways that converge on Elk-1 to control production of matrix metalloproteinase-13 by human adult articular chondrocytes.J Biol Chem. 2007 Oct 26;282(43):31409-21. doi: 10.1074/jbc.M706508200. Epub 2007 Aug 27. J Biol Chem. 2007. PMID: 17724016
-
Basic fibroblast growth factor accelerates matrix degradation via a neuro-endocrine pathway in human adult articular chondrocytes.J Cell Physiol. 2008 May;215(2):452-63. doi: 10.1002/jcp.21317. J Cell Physiol. 2008. Retraction in: J Cell Physiol. 2024 Dec;239(12):e31453. doi: 10.1002/jcp.31453. PMID: 17960584 Free PMC article. Retracted.
-
MyD88, IRAK1 and TRAF6 knockdown in human chondrocytes inhibits interleukin-1-induced matrix metalloproteinase-13 gene expression and promoter activity by impairing MAP kinase activation.Cell Signal. 2007 Dec;19(12):2549-57. doi: 10.1016/j.cellsig.2007.08.013. Epub 2007 Aug 25. Cell Signal. 2007. PMID: 17905570
-
Fibroblast growth factor-2 promotes catabolism via FGFR1-Ras-Raf-MEK1/2-ERK1/2 axis that coordinates with the PKCδ pathway in human articular chondrocytes.J Cell Biochem. 2012 Sep;113(9):2856-65. doi: 10.1002/jcb.24160. J Cell Biochem. 2012. Retraction in: J Cell Biochem. 2024 Dec;125(12):e30665. doi: 10.1002/jcb.30665. PMID: 22488450 Free PMC article. Retracted.
-
Inhibition of interleukin-1-stimulated MAP kinases, activating protein-1 (AP-1) and nuclear factor kappa B (NF-kappa B) transcription factors down-regulates matrix metalloproteinase gene expression in articular chondrocytes.Matrix Biol. 2002 Apr;21(3):251-62. doi: 10.1016/s0945-053x(02)00007-0. Matrix Biol. 2002. PMID: 12009331
Cited by
-
Growth factor transgenes interactively regulate articular chondrocytes.J Cell Biochem. 2013 Apr;114(4):908-19. doi: 10.1002/jcb.24430. J Cell Biochem. 2013. PMID: 23097312 Free PMC article.
-
Calcitonin inhibits intervertebral disc degeneration by regulating protein kinase C.J Cell Mol Med. 2020 Aug;24(15):8650-8661. doi: 10.1111/jcmm.15496. Epub 2020 Jun 21. J Cell Mol Med. 2020. PMID: 32564456 Free PMC article.
-
The synovial microenvironment of osteoarthritic joints alters RNA-seq expression profiles of human primary articular chondrocytes.Gene. 2016 Oct 15;591(2):456-64. doi: 10.1016/j.gene.2016.06.063. Epub 2016 Jul 1. Gene. 2016. PMID: 27378743 Free PMC article.
-
Role of sox9 in growth factor regulation of articular chondrocytes.J Cell Biochem. 2015 Jul;116(7):1391-400. doi: 10.1002/jcb.25099. J Cell Biochem. 2015. PMID: 25708223 Free PMC article.
-
Osteoarthritis: pathogenic signaling pathways and therapeutic targets.Signal Transduct Target Ther. 2023 Feb 3;8(1):56. doi: 10.1038/s41392-023-01330-w. Signal Transduct Target Ther. 2023. PMID: 36737426 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

