Matrix-specific p21-activated kinase activation regulates vascular permeability in atherogenesis
- PMID: 17312022
- PMCID: PMC2064028
- DOI: 10.1083/jcb.200609008
Matrix-specific p21-activated kinase activation regulates vascular permeability in atherogenesis
Abstract
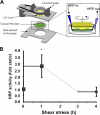
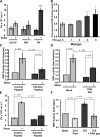
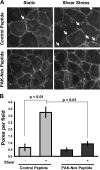
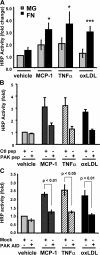
Elevated permeability of the endothelium is thought to be crucial in atherogenesis because it allows circulating lipoproteins to access subendothelial monocytes. Both local hemodynamics and cytokines may govern endothelial permeability in atherosclerotic plaque. We recently found that p21-activated kinase (PAK) regulates endothelial permeability. We now report that onset of fluid flow, atherogenic flow profiles, oxidized LDL, and proatherosclerotic cytokines all stimulate PAK phosphorylation and recruitment to cell-cell junctions. Activation of PAK is higher in cells plated on fibronectin (FN) compared to basement membrane proteins in all cases. In vivo, PAK is activated in atherosclerosis-prone regions of arteries and correlates with FN in the subendothelium. Inhibiting PAK in vivo reduces permeability in atherosclerosis-prone regions. Matrix-specific PAK activation therefore mediates elevated vascular permeability in atherogenesis.
Figures




Similar articles
-
The subendothelial extracellular matrix modulates JNK activation by flow.Circ Res. 2009 Apr 24;104(8):995-1003. doi: 10.1161/CIRCRESAHA.108.186486. Epub 2009 Mar 12. Circ Res. 2009. PMID: 19286608 Free PMC article.
-
Induction of vascular permeability: beta PIX and GIT1 scaffold the activation of extracellular signal-regulated kinase by PAK.Mol Biol Cell. 2007 Jun;18(6):2346-55. doi: 10.1091/mbc.e06-07-0584. Epub 2007 Apr 11. Mol Biol Cell. 2007. PMID: 17429073 Free PMC article.
-
Matrix-specific protein kinase A signaling regulates p21-activated kinase activation by flow in endothelial cells.Circ Res. 2010 Apr 30;106(8):1394-403. doi: 10.1161/CIRCRESAHA.109.210286. Epub 2010 Mar 11. Circ Res. 2010. PMID: 20224042 Free PMC article.
-
Vascular endothelium in atherosclerosis.Cell Tissue Res. 2009 Jan;335(1):191-203. doi: 10.1007/s00441-008-0678-5. Epub 2008 Sep 17. Cell Tissue Res. 2009. PMID: 18797930 Review.
-
PAK-in' up cGMP for the move.Cell. 2007 Jan 26;128(2):237-8. doi: 10.1016/j.cell.2007.01.002. Cell. 2007. PMID: 17254961 Review.
Cited by
-
Control of lung vascular permeability and endotoxin-induced pulmonary oedema by changes in extracellular matrix mechanics.Nat Commun. 2013;4:1759. doi: 10.1038/ncomms2774. Nat Commun. 2013. PMID: 23612300
-
α5β1 integrin signaling mediates oxidized low-density lipoprotein-induced inflammation and early atherosclerosis.Arterioscler Thromb Vasc Biol. 2014 Jul;34(7):1362-73. doi: 10.1161/ATVBAHA.114.303863. Epub 2014 May 15. Arterioscler Thromb Vasc Biol. 2014. PMID: 24833794 Free PMC article.
-
In Development-A New Paradigm for Understanding Vascular Disease.J Cardiovasc Pharmacol. 2017 May;69(5):248-263. doi: 10.1097/FJC.0000000000000480. J Cardiovasc Pharmacol. 2017. PMID: 28328747 Free PMC article. Review.
-
Blood Brothers: Hemodynamics and Cell-Matrix Interactions in Endothelial Function.Antioxid Redox Signal. 2016 Sep 1;25(7):415-34. doi: 10.1089/ars.2015.6525. Epub 2016 Feb 19. Antioxid Redox Signal. 2016. PMID: 26715135 Free PMC article. Review.
-
The subendothelial extracellular matrix modulates JNK activation by flow.Circ Res. 2009 Apr 24;104(8):995-1003. doi: 10.1161/CIRCRESAHA.108.186486. Epub 2009 Mar 12. Circ Res. 2009. PMID: 19286608 Free PMC article.
References
-
- Blackman, B.R., G. Garcia-Cardena, and M.A. Gimbrone Jr. 2002. A new in vitro model to evaluate differential responses of endothelial cells to simulated arterial shear stress waveforms. J. Biomech. Eng. 124:397–407. - PubMed
-
- Bokoch, G.M. 2003. Biology of the p21-activated kinases. Annu. Rev. Biochem. 72:743–781. - PubMed
-
- Brooks, A.R., P.I. Lelkes, and G.M. Rubanyi. 2004. Gene expression profiling of vascular endothelial cells exposed to fluid mechanical forces: relevance for focal susceptibility to atherosclerosis. Endothelium. 11:45–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

