Requirement for a complex of Wiskott-Aldrich syndrome protein (WASP) with WASP interacting protein in podosome formation in macrophages
- PMID: 17312144
- PMCID: PMC1855218
- DOI: 10.4049/jimmunol.178.5.2987
Requirement for a complex of Wiskott-Aldrich syndrome protein (WASP) with WASP interacting protein in podosome formation in macrophages
Abstract
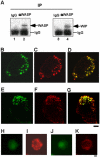
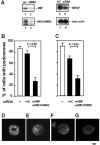
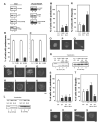
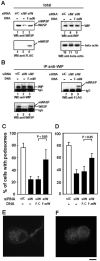
Chemotactic migration of macrophages is critical for the recruitment of leukocytes to inflamed tissues. Macrophages use a specialized adhesive structure called a podosome to migrate. Podosome formation requires the Wiskott-Aldrich syndrome protein (WASP), which is a product of the gene defective in an X-linked inherited immunodeficiency disorder, the Wiskott-Aldrich syndrome. Macrophages from WASP-deficient Wiskott-Aldrich syndrome patients lack podosomes, resulting in defective chemotactic migration. However, the molecular basis for podosome formation is not fully understood. I have shown that the WASP interacting protein (WIP), a binding partner of WASP, plays an important role in podosome formation in macrophages. I showed that WASP bound WIP to form a complex at podosomes and that the knockdown of WIP impairs podosome formation. When WASP binding to WIP was blocked, podosome formation was also impaired. When WASP expression was reduced by small interfering RNA transfection, the amount of the complex of WASP with WIP decreased, resulting in reduced podosome formation. Podosomes were restored by reconstitution of the WASP-WIP complex in WASP knockdown cells. These results indicate that the WASP-WIP complex is required for podosome formation in macrophages. When podosome formation was reduced by blocking WASP binding to WIP, transendothelial migration of macrophages, the most crucial process in macrophage trafficking, was impaired. These results suggest that a complex of WASP with WIP plays a critical role in podosome formation, thereby mediating efficient transendothelial migration of macrophages.
Figures

Similar articles
-
FBP17 Mediates a Common Molecular Step in the Formation of Podosomes and Phagocytic Cups in Macrophages.J Biol Chem. 2009 Mar 27;284(13):8548-56. doi: 10.1074/jbc.M805638200. Epub 2009 Jan 20. J Biol Chem. 2009. PMID: 19155218 Free PMC article.
-
A complex of Wiskott-Aldrich syndrome protein with mammalian verprolins plays an important role in monocyte chemotaxis.J Immunol. 2006 Jun 1;176(11):6576-85. doi: 10.4049/jimmunol.176.11.6576. J Immunol. 2006. PMID: 16709815
-
Wiskott-Aldrich syndrome protein is a key regulator of the phagocytic cup formation in macrophages.J Biol Chem. 2007 Nov 23;282(47):34194-203. doi: 10.1074/jbc.M705999200. Epub 2007 Sep 21. J Biol Chem. 2007. PMID: 17890224
-
WIP remodeling actin behind the scenes: how WIP reshapes immune and other functions.Int J Mol Sci. 2012;13(6):7629-7647. doi: 10.3390/ijms13067629. Epub 2012 Jun 21. Int J Mol Sci. 2012. PMID: 22837718 Free PMC article. Review.
-
WASP-WIP complex in the molecular pathogenesis of Wiskott-Aldrich syndrome.Pediatr Int. 2016 Jan;58(1):4-7. doi: 10.1111/ped.12819. Epub 2015 Dec 5. Pediatr Int. 2016. PMID: 26331277 Review.
Cited by
-
Fascin1 promotes cell migration of mature dendritic cells.J Immunol. 2011 Mar 1;186(5):2850-9. doi: 10.4049/jimmunol.1001667. Epub 2011 Jan 24. J Immunol. 2011. PMID: 21263068 Free PMC article.
-
The diverse functions of Src family kinases in macrophages.Front Biosci. 2008 May 1;13:4426-50. doi: 10.2741/3015. Front Biosci. 2008. PMID: 18508521 Free PMC article. Review.
-
Antitumor Activity of a Novel LAIR1 Antagonist in Combination with Anti-PD1 to Treat Collagen-Rich Solid Tumors.Mol Cancer Ther. 2024 Aug 1;23(8):1144-1158. doi: 10.1158/1535-7163.MCT-23-0866. Mol Cancer Ther. 2024. PMID: 38648067 Free PMC article.
-
Actin cytoskeletal defects in immunodeficiency.Immunol Rev. 2013 Nov;256(1):282-99. doi: 10.1111/imr.12114. Immunol Rev. 2013. PMID: 24117828 Free PMC article. Review.
-
Macrophage Migration and Its Regulation by CSF-1.Int J Cell Biol. 2012;2012:501962. doi: 10.1155/2012/501962. Epub 2012 Feb 15. Int J Cell Biol. 2012. PMID: 22505929 Free PMC article.
References
-
- Wiskott A. Familiarer, angeborener Morbus Welhofii? Monatsschr Kinderheilkd. 1937;68:212–216.
-
- Aldrich RA, Steinberg AG, Campbell DC. Pedigree demonstrating a sex-linked recessive condition characterized by draining ears, eczematoid dermatitis and bloody diarrhea. Pediatrics. 1954;13:133–139. - PubMed
-
- Ochs HD, Thrasher AJ. The Wiskott-Aldrich syndrome.[see comment] Journal of Allergy & Clinical Immunology. 2006;117:725–738. quiz 739. - PubMed
-
- Derry JM, Ochs HD, Francke U. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. [Published erratum appears in 1994 Cell 79: following 922.] Cell. 1994;78:635–644. - PubMed
-
- Burns S, Cory GO, Vainchenker W, Thrasher AJ. Mechanisms of WASp-mediated hematologic and immunologic disease. Blood. 2004;104:3454–3462. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

