Requirement of KISS1 secretion for multiple organ metastasis suppression and maintenance of tumor dormancy
- PMID: 17312308
- PMCID: PMC1820615
- DOI: 10.1093/jnci/djk053
Requirement of KISS1 secretion for multiple organ metastasis suppression and maintenance of tumor dormancy
Erratum in
- J Natl Cancer Inst. 2007 Jul 20;99(12):977
Abstract
Background: The KISS1 protein suppresses metastasis of several tumor models without blocking orthotopic tumor growth, but the mechanism remains elusive. For its role in human sexual maturation, KISS1 protein is secreted and processed to kisspeptins, which bind to the G protein-coupled receptor GPR54. We tested the hypothesis that KISS1 secretion is required for metastasis suppression via GPR54.
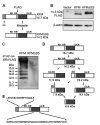
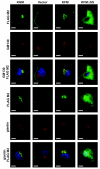
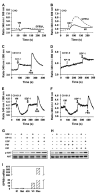
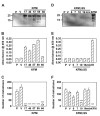
Methods: KISS1 containing an internal FLAG epitope with (KFM) or without (KFMdeltaSS) a signal sequence was transfected into C8161.9 human melanoma cells, which do not express endogenous KISS1. Whole-cell lysates and conditioned medium from C8161.9(KFM) and C8161.9(KFMdeltaSS) cells were collected and analyzed for kisspeptins by immunoprecipitation and enzyme-linked immunosorbent assay. GPR54 levels were measured using real-time reverse transcription-polymerase chain reaction. The ability of conditioned medium from C8161.9(KFM) and C8161.9(KFMdeltaSS) cells to stimulate calcium mobilization in GPR54-expressing Chinese hamster ovary cells (CHO-G) and in C8161.9 cells was evaluated. Metastasis was monitored in athymic mice (groups of 10 per experiment) that were injected with C8161.9(KFM) or C8161.9(KFMdeltaSS) cells labeled with enhanced green fluorescent protein. Survival of mice injected with C8161.9 or C8161.9(KFM) cells was analyzed by Kaplan-Meier methods.
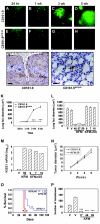
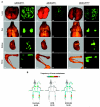
Results: Full-length KFM and KFMdeltaSS were detected in whole-cell lysates of C8161.9(KFM) and C8161.9(KFMdeltaSS) cells, respectively, but kisspeptins were detected only in conditioned medium of C8161.9(KFM) cells. In vivo, C8161.9(KFM), but not C8161.9(KFMdeltaSS), cells were suppressed for metastasis to lung, eye, kidney, and bone, with corresponding differences in mouse survival (median > 120 versus 42 days). C8161.9(KFM) cells seeded mouse lungs but did not form macroscopic metastases. Conditioned medium from C8161.9(KFM), but not C8161.9(KFMdeltaSS), cells stimulated calcium mobilization in CHO-G cells. GPR54 expression was low in C8161.9 cells, which were not stimulated by conditioned medium from C8161.9(KFM) cells.
Conclusions: KISS1 secretion was required for multiple organ metastasis suppression and for maintenance of disseminated cells in a dormant state. The absence of GPR54 expression in C8161.9 cells (whose metastatic spread was suppressed by KFM) suggests that metastasis suppression is not mediated through this receptor. The results imply the existence of another KISS1 receptor and/or paracrine signaling. The findings raise the possibility that soluble KISS1, kisspeptins, or mimetics could be used to maintain tumor dormancy, rendering treatment of already disseminated tumor cells (i.e., micrometastases) a legitimate target.
Figures
References
-
- Jiang Y, Berk M, Singh LS, Tan HY, Yin LH, Powell CT, et al. KiSS1 suppresses metastasis in human ovarian cancer via inhibition of protein kinase C alpha. Clin Exp Metastasis. 2005;22:369–76. - PubMed
-
- Lee J-H, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88:1731–7. - PubMed
-
- Lee J-H, Welch DR. Identification of highly expressed genes in metastasis-suppressed chromosome 6/human malignant melanoma hybrid cells using subtractive hybridization and differential display. Int J Cancer. 1997;71:1035–44. - PubMed
-
- Lee J-H, Welch DR. Suppression of metastasis in human breast carcinoma MDA-MB-435 cells after transfection with the metastasis suppressor gene, KiSS-1. Cancer Res. 1997;57:2384–7. - PubMed
-
- Lee J-H, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene [erratum] J Natl Cancer Inst. 1997;89:1549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

