Nucleation and stability of hydrogen-bond surrogate-based alpha-helices
- PMID: 17312961
- PMCID: PMC1807155
- DOI: 10.1039/b612891b
Nucleation and stability of hydrogen-bond surrogate-based alpha-helices
Abstract
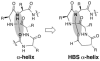
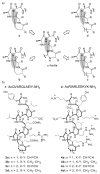
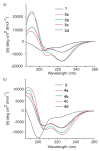
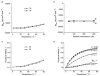
We have reported a new class of artificial alpha-helices in which a pre-organized alpha-turn nucleates the helical conformation [R. N. Chapman, G. Dimartino, and P. S. Arora, J Am. Chem. Soc., 2004, 126, 12252 and D. Wang, K. Chen, J. L. Kulp, III, and P. S. Arora, J. Am. Chem. Soc., 2006, 128, 9248]. This manuscript describes the effect of the core nucleation template on the overall helicity of the peptides and demonstrates that the macrocycle which most closely mimics the 13-membered hydrogen-bonded alpha-turn in canonical alpha-helices also affords the most stable artificial alpha-helix. We also investigate the stability of these synthetic helices through classical helix-coil parameters and find that the denaturation behavior of HBS alpha-helices agrees with the theoretical properties of a peptide with a well-defined and stable helix nucleus.
Figures


Similar articles
-
A hydrogen bond surrogate approach for stabilization of short peptide sequences in alpha-helical conformation.Acc Chem Res. 2008 Oct;41(10):1289-300. doi: 10.1021/ar700264k. Epub 2008 Jul 17. Acc Chem Res. 2008. PMID: 18630933 Free PMC article.
-
Evaluation of biologically relevant short alpha-helices stabilized by a main-chain hydrogen-bond surrogate.J Am Chem Soc. 2006 Jul 19;128(28):9248-56. doi: 10.1021/ja062710w. J Am Chem Soc. 2006. PMID: 16834399 Free PMC article.
-
Atomic structure of a short alpha-helix stabilized by a main chain hydrogen-bond surrogate.J Am Chem Soc. 2008 Apr 2;130(13):4334-7. doi: 10.1021/ja077704u. Epub 2008 Mar 11. J Am Chem Soc. 2008. PMID: 18331030
-
Protein Domain Mimics as Modulators of Protein-Protein Interactions.Acc Chem Res. 2017 Jun 20;50(6):1313-1322. doi: 10.1021/acs.accounts.7b00130. Epub 2017 May 31. Acc Chem Res. 2017. PMID: 28561588 Free PMC article. Review.
-
Contemporary strategies for the stabilization of peptides in the alpha-helical conformation.Curr Opin Chem Biol. 2008 Dec;12(6):692-7. doi: 10.1016/j.cbpa.2008.08.019. Epub 2008 Sep 13. Curr Opin Chem Biol. 2008. PMID: 18793750 Free PMC article. Review.
Cited by
-
Nucleation effects in peptide foldamers.J Am Chem Soc. 2012 Jul 18;134(28):11495-502. doi: 10.1021/ja301953j. Epub 2012 Jul 5. J Am Chem Soc. 2012. PMID: 22715982 Free PMC article.
-
A hydrogen bond surrogate approach for stabilization of short peptide sequences in alpha-helical conformation.Acc Chem Res. 2008 Oct;41(10):1289-300. doi: 10.1021/ar700264k. Epub 2008 Jul 17. Acc Chem Res. 2008. PMID: 18630933 Free PMC article.
-
Hydrogen Bond Surrogate Stabilization of β-Hairpins.ACS Chem Biol. 2018 Aug 17;13(8):2027-2032. doi: 10.1021/acschembio.8b00641. Epub 2018 Jul 18. ACS Chem Biol. 2018. PMID: 30005156 Free PMC article.
-
Structural and pharmacological effects of ring-closing metathesis in peptides.Molecules. 2010 Sep 21;15(9):6638-77. doi: 10.3390/molecules15096638. Molecules. 2010. PMID: 20877250 Free PMC article. Review.
-
Design, synthesis and protein-targeting properties of thioether-linked hydrogen bond surrogate helices.Chem Commun (Camb). 2012 Feb 1;48(10):1416-8. doi: 10.1039/c1cc14730g. Epub 2011 Sep 28. Chem Commun (Camb). 2012. PMID: 21952530 Free PMC article.
References
-
- Chapman RN, Dimartino G, Arora PS. J Am Cem Soc. 2004;126:12252–12253. - PubMed
-
- Lifson S, Roig AJ. Cem Pys. 1961;34:1963–1974.
-
- Zimm BH, Bragg JK. J Cem Pys. 1959;31:526–535.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

