Nucleation and stability of hydrogen-bond surrogate-based alpha-helices
- PMID: 17312961
- PMCID: PMC1807155
- DOI: 10.1039/b612891b
Nucleation and stability of hydrogen-bond surrogate-based alpha-helices
Abstract
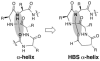
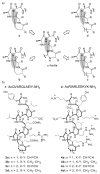
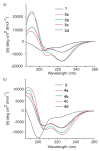
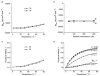
We have reported a new class of artificial alpha-helices in which a pre-organized alpha-turn nucleates the helical conformation [R. N. Chapman, G. Dimartino, and P. S. Arora, J Am. Chem. Soc., 2004, 126, 12252 and D. Wang, K. Chen, J. L. Kulp, III, and P. S. Arora, J. Am. Chem. Soc., 2006, 128, 9248]. This manuscript describes the effect of the core nucleation template on the overall helicity of the peptides and demonstrates that the macrocycle which most closely mimics the 13-membered hydrogen-bonded alpha-turn in canonical alpha-helices also affords the most stable artificial alpha-helix. We also investigate the stability of these synthetic helices through classical helix-coil parameters and find that the denaturation behavior of HBS alpha-helices agrees with the theoretical properties of a peptide with a well-defined and stable helix nucleus.
Figures


References
-
- Chapman RN, Dimartino G, Arora PS. J Am Cem Soc. 2004;126:12252–12253. - PubMed
-
- Lifson S, Roig AJ. Cem Pys. 1961;34:1963–1974.
-
- Zimm BH, Bragg JK. J Cem Pys. 1959;31:526–535.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

