Impaired spatial working memory but spared spatial reference memory following functional loss of NMDA receptors in the dentate gyrus
- PMID: 17313573
- PMCID: PMC2777262
- DOI: 10.1111/j.1460-9568.2007.05312.x
Impaired spatial working memory but spared spatial reference memory following functional loss of NMDA receptors in the dentate gyrus
Abstract
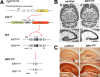
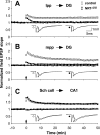
Novel spatially restricted genetic manipulations can be used to assess contributions made by synaptic plasticity to learning and memory, not just selectively within the hippocampus, but even within specific hippocampal subfields. Here we generated genetically modified mice (NR1(deltaDG) mice) exhibiting complete loss of the NR1 subunit of the N-methyl-D-aspartate receptor specifically in the granule cells of the dentate gyrus. There was no evidence of any reduction in NR1 subunit levels in any of the other hippocampal subfields, or elsewhere in the brain. NR1(deltaDG) mice displayed severely impaired long-term potentiation (LTP) in both medial and lateral perforant path inputs to the dentate gyrus, whereas LTP was unchanged in CA3-to-CA1 cell synapses in hippocampal slices. Behavioural assessment of NR1(deltaDG) mice revealed a spatial working memory impairment on a three-from-six radial arm maze task despite normal hippocampus-dependent spatial reference memory acquisition and performance of the same task. This behavioural phenotype resembles that of NR1(deltaCA3) mice but differs from that of NR1(deltaCA1) mice which do show a spatial reference memory deficit, consistent with the idea of subfield-specific contributions to hippocampal information processing. Furthermore, this pattern of selective functional loss and sparing is the same as previously observed with the global GluR-A L-alpha-amino-3-hydroxy-5-methyl-4-isoxazelopropionate receptor subunit knockout, a mutation which blocks the expression of hippocampal LTP. The present results show that dissociations between spatial working memory and spatial reference memory can be induced by disrupting synaptic plasticity specifically and exclusively within the dentate gyrus subfield of the hippocampal formation.
Figures


References
-
- Bannerman DM, Good MA, Butcher SP, Ramsay M, Morris RGM. Distinct components of spatial learning revealed by prior training and NMDA receptor blockade. Nature. 1995;378:182–186. - PubMed
-
- Bannerman DM, Rawlins JNP, Good MA. The drugs don't work – or do they? a review of pharmacological studies assessing the contribution of NMDA receptors to hippocampal-dependent forms of memory. Psychopharmacology. 2006;188:552–566. - PubMed
-
- Bannerman DM, Rawlins JNP, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus-memory and anxiety. Neurosci.Biobehav.Rev. 2004;28:273–283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

