Simian immunodeficiency virus (SIV) infection influences the level and function of regulatory T cells in SIV-infected rhesus macaques but not SIV-infected sooty mangabeys
- PMID: 17314162
- PMCID: PMC1900154
- DOI: 10.1128/JVI.00026-07
Simian immunodeficiency virus (SIV) infection influences the level and function of regulatory T cells in SIV-infected rhesus macaques but not SIV-infected sooty mangabeys
Abstract
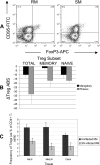
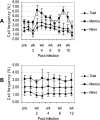
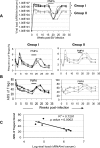
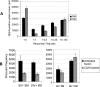
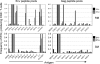
Differences in clinical outcome of simian immunodeficiency virus (SIV) infection in disease-resistant African sooty mangabeys (SM) and disease-susceptible Asian rhesus macaques (RM) prompted us to examine the role of regulatory T cells (Tregs) in these two animal models. Results from a cross-sectional study revealed maintenance of the frequency and absolute number of peripheral Tregs in chronically SIV-infected SM while a significant loss occurred in chronically SIV-infected RM compared to uninfected animals. A longitudinal study of experimentally SIV-infected animals revealed a transient increase in the frequency of Tregs from baseline values following acute infection in RM, but no change in the frequency of Tregs occurred in SM during this period. Further examination revealed a strong correlation between plasma viral load (VL) and the level of Tregs in SIV-infected RM but not SM. A correlation was also noted in SIV-infected RM that control VL spontaneously or in response to antiretroviral chemotherapy. In addition, immunofluorescent cell count assays showed that while Treg-depleted peripheral blood mononuclear cells from RM led to a significant enhancement of CD4+ and CD8+ T-cell responses to select pools of SIV peptides, there was no detectable T-cell response to the same pool of SIV peptides in Treg-depleted cells from SIV-infected SM. Our data collectively suggest that while Tregs do appear to play a role in the control of viremia and the magnitude of the SIV-specific immune response in RM, their role in disease resistance in SM remains unclear.
Figures

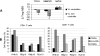
Similar articles
-
Differential CD4+ T-lymphocyte apoptosis and bystander T-cell activation in rhesus macaques and sooty mangabeys during acute simian immunodeficiency virus infection.J Virol. 2009 Jan;83(2):572-83. doi: 10.1128/JVI.01715-08. Epub 2008 Nov 5. J Virol. 2009. PMID: 18987149 Free PMC article.
-
Immunological and virological studies of natural SIV infection of disease-resistant nonhuman primates.Immunol Lett. 1996 Jun;51(1-2):59-68. doi: 10.1016/0165-2478(96)02556-4. Immunol Lett. 1996. PMID: 8811346
-
Reduced Simian Immunodeficiency Virus Replication in Macrophages of Sooty Mangabeys Is Associated with Increased Expression of Host Restriction Factors.J Virol. 2015 Oct;89(20):10136-44. doi: 10.1128/JVI.00710-15. Epub 2015 Jul 22. J Virol. 2015. PMID: 26202248 Free PMC article.
-
A case for innate immune effector mechanisms as contributors to disease resistance in SIV-infected sooty mangabeys.Curr HIV Res. 2009 Jan;7(1):12-22. doi: 10.2174/157016209787048465. Curr HIV Res. 2009. PMID: 19149550 Review.
-
Naturally SIV-infected sooty mangabeys: are we closer to understanding why they do not develop AIDS?J Med Primatol. 2005 Oct;34(5-6):243-52. doi: 10.1111/j.1600-0684.2005.00122.x. J Med Primatol. 2005. PMID: 16128919 Review.
Cited by
-
Macaque models of enhanced susceptibility to HIV.Virol J. 2015 Jun 14;12:90. doi: 10.1186/s12985-015-0320-6. Virol J. 2015. PMID: 26070461 Free PMC article. Review.
-
Partial regulatory T cell depletion prior to acute feline immunodeficiency virus infection does not alter disease pathogenesis.PLoS One. 2011 Feb 25;6(2):e17183. doi: 10.1371/journal.pone.0017183. PLoS One. 2011. PMID: 21364928 Free PMC article.
-
Translational mini-review series on Th17 cells: induction of interleukin-17 production by regulatory T cells.Clin Exp Immunol. 2010 Feb;159(2):120-30. doi: 10.1111/j.1365-2249.2009.04038.x. Epub 2009 Nov 11. Clin Exp Immunol. 2010. PMID: 19912251 Free PMC article. Review.
-
Regulatory T cells in arterivirus and coronavirus infections: do they protect against disease or enhance it?Viruses. 2012 May;4(5):833-46. doi: 10.3390/v4050833. Epub 2012 May 15. Viruses. 2012. PMID: 22754651 Free PMC article. Review.
-
Relationship between regulatory T cells and immune activation in human immunodeficiency virus-infected patients interrupting antiretroviral therapy.PLoS One. 2010 Jul 21;5(7):e11659. doi: 10.1371/journal.pone.0011659. PLoS One. 2010. PMID: 20657770 Free PMC article. Clinical Trial.
References
-
- Annacker, O., R. Pimenta-Araujo, O. Burlen-Defranoux, T. C. Barbosa, A. Cumano, and A. Bandeira. 2001. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J. Immunol. 166:3008-3018. - PubMed
-
- Apoil, P. A., B. Puissant, F. Roubinet, M. Abbal, P. Massip, and A. Blancher. 2005. FoxP3 mRNA levels are decreased in peripheral blood CD4+ lymphocytes from HIV-positive patients. J. Acquir. Immune Defic. Syndr. 39:381-385. - PubMed
-
- Baecher-Allan, C., J. A. Brown, G. J. Freeman, and D. A. Hafler. 2001. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167:1245-1253. - PubMed
-
- Baecher-Allan, C., E. Wolf, and D. A. Hafler. 2005. Functional analysis of highly defined, FACS-isolated populations of human regulatory CD4+CD25+ T cells. Clin. Immunol. 115:10-18. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

