Kaposi's sarcoma-associated herpesvirus LANA protein downregulates nuclear glycogen synthase kinase 3 activity and consequently blocks differentiation
- PMID: 17314169
- PMCID: PMC1900136
- DOI: 10.1128/JVI.02548-06
Kaposi's sarcoma-associated herpesvirus LANA protein downregulates nuclear glycogen synthase kinase 3 activity and consequently blocks differentiation
Abstract
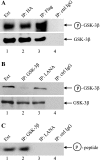
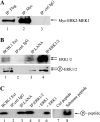
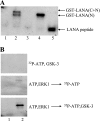
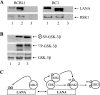
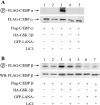
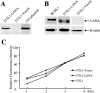
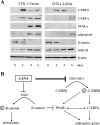
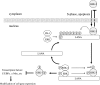
The Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen (LANA) protein interacts with glycogen synthase kinase 3 (GSK-3) and relocalizes GSK-3 in a manner that leads to stabilization of beta-catenin and upregulation of beta-catenin-responsive cell genes. The LANA-GSK-3 interaction was further examined to determine whether there were additional downstream consequences. In the present study, the nuclear GSK-3 bound to LANA in transfected cells and in BCBL1 primary effusion lymphoma cells was found to be enriched for the inactive serine 9-phosphorylated form of GSK-3. The mechanism of inactivation of nuclear GSK-3 involved LANA recruitment of the extracellular signal-regulated kinases 1 and 2 (ERK1/2) and the ribosomal S6 kinase 1 (RSK1). ERK1/2 and RSK1 coprecipitated with LANA, and LANA was a substrate for ERK1 in vitro. A model is proposed for the overall inactivation of nuclear GSK-3 that incorporates the previously described GSK-3 phosphorylation of LANA itself. Functional inactivation of nuclear GSK-3 was demonstrated by the ability of LANA to limit phosphorylation of the known GSK-3 substrates C/EBPbeta and C/EBPalpha. The effect of LANA-mediated ablation of C/EBP phosphorylation on differentiation was modeled in the well-characterized 3T3L1 adipogenesis system. LANA-expressing 3T3L1 cells were impaired in their ability to undergo differentiation and adipogenesis. C/EBPbeta induction followed the same time course as that seen in vector-transduced cells, but there was delayed and reduced induction of C/EBPbeta transcriptional targets in LANA-expressing cells. We conclude that LANA inactivates nuclear GSK-3 and modifies the function of proteins that are GSK-3 substrates. In the case of C/EBPs, this translates into LANA-mediated inhibition of differentiation.
Figures

Similar articles
-
Regulation of the interaction between glycogen synthase kinase 3 and the Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen.J Virol. 2005 Aug;79(16):10429-41. doi: 10.1128/JVI.79.16.10429-10441.2005. J Virol. 2005. PMID: 16051835 Free PMC article.
-
The Kaposi's sarcoma-associated herpesvirus LANA protein stabilizes and activates c-Myc.J Virol. 2007 Oct;81(19):10451-9. doi: 10.1128/JVI.00804-07. Epub 2007 Jul 18. J Virol. 2007. PMID: 17634226 Free PMC article.
-
The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus manipulates the activity of glycogen synthase kinase-3beta.J Virol. 2003 Jul;77(14):8019-30. doi: 10.1128/jvi.77.14.8019-8030.2003. J Virol. 2003. PMID: 12829841 Free PMC article.
-
Manipulation of glycogen-synthase kinase-3 activity in KSHV-associated cancers.J Mol Med (Berl). 2004 Apr;82(4):223-31. doi: 10.1007/s00109-003-0519-7. Epub 2004 Jan 9. J Mol Med (Berl). 2004. PMID: 14991150 Review.
-
Structure and function of latency-associated nuclear antigen.Curr Top Microbiol Immunol. 2007;312:101-36. doi: 10.1007/978-3-540-34344-8_4. Curr Top Microbiol Immunol. 2007. PMID: 17089795 Free PMC article. Review.
Cited by
-
The Ubiquitin System and Kaposi's Sarcoma-Associated Herpesvirus.Front Microbiol. 2012 Feb 23;3:66. doi: 10.3389/fmicb.2012.00066. eCollection 2012. Front Microbiol. 2012. PMID: 22375140 Free PMC article.
-
Kaposi sarcoma herpesvirus pathogenesis.Philos Trans R Soc Lond B Biol Sci. 2017 Oct 19;372(1732):20160275. doi: 10.1098/rstb.2016.0275. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28893942 Free PMC article. Review.
-
Activation of DNA Damage Response Induced by the Kaposi's Sarcoma-Associated Herpes Virus.Int J Mol Sci. 2016 Jun 1;17(6):854. doi: 10.3390/ijms17060854. Int J Mol Sci. 2016. PMID: 27258263 Free PMC article. Review.
-
Insight into the Epigenetics of Kaposi's Sarcoma-Associated Herpesvirus.Int J Mol Sci. 2023 Oct 6;24(19):14955. doi: 10.3390/ijms241914955. Int J Mol Sci. 2023. PMID: 37834404 Free PMC article. Review.
-
Phosphorylation of the chromatin binding domain of KSHV LANA.PLoS Pathog. 2012;8(10):e1002972. doi: 10.1371/journal.ppat.1002972. Epub 2012 Oct 18. PLoS Pathog. 2012. PMID: 23093938 Free PMC article.
References
-
- An, F. Q., N. Compitello, E. Horwitz, M. Sramkoski, E. S. Knudsen, and R. Renne. 2005. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus modulates cellular gene expression and protects lymphoid cells from P16INK4A-induced cell cycle arrest. J. Biol. Chem. 280:3862-3874. - PubMed
-
- Ballestas, M. E., P. A. Chatis, and K. M. Kaye. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641-644. - PubMed
-
- Barak, Y., M. C. Nelson, E. S. Ong, Y. Z. Jones, P. Ruiz-Lozano, K. R. Chien, A. Koder, and R. M. Evans. 1999. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol. Cell 4:585-595. - PubMed
-
- Barbera, A. J., J. V. Chodaparambil, B. Kelley-Clarke, V. Joukov, J. C. Walter, K. Luger, and K. M. Kaye. 2006. The nucleosomal surface as a docking station for Kaposi's sarcoma herpesvirus LANA. Science 311:856-861. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

