Primary CD4+ T-cell responses provide both helper and cytotoxic functions during Epstein-Barr virus infection and transformation of fetal cord blood B cells
- PMID: 17314172
- PMCID: PMC1900140
- DOI: 10.1128/JVI.02608-06
Primary CD4+ T-cell responses provide both helper and cytotoxic functions during Epstein-Barr virus infection and transformation of fetal cord blood B cells
Abstract
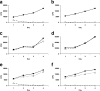
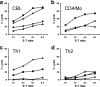
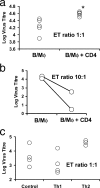
Most humans carry Epstein-Barr virus (EBV) in circulating memory B cells as a latent infection that is controlled by an immune response. When infected by EBV, B lymphocytes in fetal cord blood are readily transformed to lymphoblastoid cell lines (LCL). It is frequently assumed that this high efficiency of transformation is due to the absence of a primary immune response. However, cord blood lymphocytes stimulated with autologous LCL yield CD4+ T cells that can completely inhibit the growth of LCL by a major histocompatibility complex-restricted cytotoxic mechanism mediated by granulysin and granzyme B. Because EBV-transformed B cells maintain the phenotype of antigen-activated B-cell blasts, they can potentially receive inhibitory or helper functions from CD4+ T cells. To assess these functions, the effect of EBV-specific CD4+ T cells on the efficiency of virus transformation of autologous B cells was assayed. Paradoxically, although the cytotoxic CD4+ T-cell lines reduced EBV B-cell transformation at a high effector/target ratio of 10:1, they caused a twofold increase in B-cell transformation at the lower effector/target ratio of 1:1. Th1-polarized CD4+ T cells were more effective at inhibiting B-cell transformation, but Th2-polarized cell lines had reduced cytotoxic activity, were unable to inhibit LCL growth, and caused a 10-fold increase in transformation efficiency. Tonsil lymphoid follicles lacked NK cells and CD8+ T cells but contained CD4+ T cells. We propose that CD4+ T cells provide helper or cytotoxic functions to EBV-transformed B cells and that the balance of these functions within tonsil compartments is critical in establishing asymptomatic primary EBV infection and maintaining a stable lifelong latent infection.
Figures



Similar articles
-
Primary immune responses by cord blood CD4(+) T cells and NK cells inhibit Epstein-Barr virus B-cell transformation in vitro.J Virol. 2002 May;76(10):5071-81. doi: 10.1128/jvi.76.10.5071-5081.2002. J Virol. 2002. PMID: 11967323 Free PMC article.
-
Control of Epstein-Barr virus infection in vitro by T helper cells specific for virion glycoproteins.J Exp Med. 2006 Apr 17;203(4):995-1006. doi: 10.1084/jem.20051287. Epub 2006 Mar 20. J Exp Med. 2006. PMID: 16549597 Free PMC article.
-
In vitro cytokine production and growth inhibition of lymphoblastoid cell lines by CD4+ T cells from Epstein-Barr virus (EBV) seropositive donors.Clin Exp Immunol. 2001 Oct;126(1):101-10. doi: 10.1046/j.1365-2249.2001.01641.x. Clin Exp Immunol. 2001. PMID: 11678905 Free PMC article.
-
Regulation and dysregulation of Epstein-Barr virus latency: implications for the development of autoimmune diseases.Autoimmunity. 2008 May;41(4):298-328. doi: 10.1080/08916930802024772. Autoimmunity. 2008. PMID: 18432410 Review.
-
Immunodominance of lytic cycle antigens in Epstein-Barr virus-specific CD4+ T cell preparations for therapy.PLoS One. 2007 Jul 4;2(7):e583. doi: 10.1371/journal.pone.0000583. PLoS One. 2007. PMID: 17611619 Free PMC article. Review.
Cited by
-
Fighting Viral Infections and Virus-Driven Tumors with Cytotoxic CD4+ T Cells.Front Immunol. 2017 Feb 27;8:197. doi: 10.3389/fimmu.2017.00197. eCollection 2017. Front Immunol. 2017. PMID: 28289418 Free PMC article. Review.
-
LMP1-deficient Epstein-Barr virus mutant requires T cells for lymphomagenesis.J Clin Invest. 2015 Jan;125(1):304-15. doi: 10.1172/JCI76357. Epub 2014 Dec 8. J Clin Invest. 2015. PMID: 25485679 Free PMC article.
-
A study of Epstein-Barr virus infection in the Chinese tree shrew(Tupaia belangeri chinensis).Virol J. 2017 Oct 6;14(1):193. doi: 10.1186/s12985-017-0859-5. Virol J. 2017. PMID: 28985762 Free PMC article.
-
Patients with Epstein Barr virus-positive lymphomas have decreased CD4(+) T-cell responses to the viral nuclear antigen 1.Int J Cancer. 2008 Dec 15;123(12):2824-31. doi: 10.1002/ijc.23845. Int J Cancer. 2008. PMID: 18781564 Free PMC article.
-
Characterization of Drug-Specific CD4+ T-Cells Reveals Possible Roles of HLA Class II in the Pathogenesis of Carbamazepine Hypersensitivity Reactions.Chem Res Toxicol. 2023 May 15;36(5):757-768. doi: 10.1021/acs.chemrestox.2c00414. Epub 2023 Apr 19. Chem Res Toxicol. 2023. PMID: 37074725 Free PMC article.
References
-
- Babcock, G. J., D. Hochberg, and A. D. Thorley-Lawson. 2000. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity 13:497-506. - PubMed
-
- Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. - PubMed
-
- Bickham, K., and C. Munz. 2003. Contrasting roles of dendritic cells and B cells in the immune control of Epstein-Barr virus. Curr. Top. Microbiol. Immunol. 276:55-76. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

