Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation
- PMID: 17314287
- PMCID: PMC6673555
- DOI: 10.1523/JNEUROSCI.4738-06.2007
Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation
Abstract
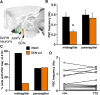
Ovulation is initiated by a surge of gonadotropin-releasing hormone (GnRH) secretion by the brain. GnRH is normally under negative feedback control by ovarian steroids. During sustained exposure to estradiol in the late follicular phase of the reproductive cycle, however, the feedback action of this steroid switches to positive, inducing the surge. Here, we used an established ovariectomized, estradiol-treated (OVX+E) mouse model exhibiting daily surges to investigate the neurobiological mechanisms underlying this switch. Specifically, we examined changes in GABA transmission to GnRH neurons, which can be excited by GABA(A) receptor activation. Spontaneous GABAergic postsynaptic currents (PSCs) were recorded in GnRH neurons from OVX+E and OVX mice in coronal and sagittal slices. There were no diurnal changes in PSC frequency in cells from OVX mice in either slice orientation. In OVX+E cells in both orientations, PSC frequency was low during negative feedback but increased at surge onset. During the surge peak, this increase subsided in coronal slices but persisted in sagittal slices. Comparison of PSCs before and during tetrodotoxin (TTX) treatment showed TTX decreased PSC frequency in OVX+E cells in sagittal slices, but not coronal slices. This indicates estradiol acts on multiple GABAergic afferent populations to increase transmission through both activity-dependent and -independent mechanisms. Estradiol also increased PSC amplitude during the surge. Estradiol and the diurnal cycle thus interact to induce shifts in both GABA transmission and postsynaptic response that would produce appropriate changes in GnRH neuron firing activity and hormone release.
Figures







References
-
- Adams VL, Goodman RL, Salm AK, Coolen LM, Karsch FJ, Lehman MN. Morphological plasticity in the neural circuitry responsible for seasonal breeding in the ewe. Endocrinology. 2006;147:4843–4851. - PubMed
-
- Barry PH. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods. 1994;51:107–116. - PubMed
-
- Beltramino C, Taleisnik S. Dual action of electrochemical stimulation of the bed nucleus of the stria terminalis on the release of LH. Neuroendocrinology. 1980;30:238–242. - PubMed
-
- Bilger M, Heger S, Brann DW, Paredes A, Ojeda SR. A conditional tetracycline-regulated increase in gamma aminobutyric acid production near luteinizing hormone-releasing hormone nerve terminals disrupts estrous cyclicity in the rat. Endocrinology. 2001;142:2102–2114. - PubMed
-
- Bless EP, Westaway WA, Schwarting GA, Tobet SA. Effects of gamma-aminobutyric acid(A) receptor manipulation on migrating gonadotropin-releasing hormone neurons through the entire migratory route in vivo and in vitro. Endocrinology. 2000;141:1254–1262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
