Apolipoprotein E-containing lipoproteins protect neurons from apoptosis via a signaling pathway involving low-density lipoprotein receptor-related protein-1
- PMID: 17314289
- PMCID: PMC6673537
- DOI: 10.1523/JNEUROSCI.5471-06.2007
Apolipoprotein E-containing lipoproteins protect neurons from apoptosis via a signaling pathway involving low-density lipoprotein receptor-related protein-1
Abstract
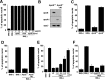
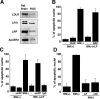
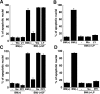
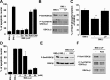
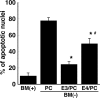
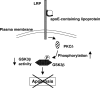
Apolipoprotein E (apoE)-containing lipoproteins (LPs) are secreted by glia and play important roles in lipid homeostasis in the CNS. Glia-derived LPs also promote synaptogenesis and stimulate axon growth of CNS neurons. Here, we provide evidence that glia-derived LPs protect CNS neurons from apoptosis by a receptor-mediated signaling pathway. The protective effect was greater for apolipoprotein E3 than for apolipoprotein E4, the expression of which is a risk factor for Alzheimer's disease. The anti-apoptotic effect of LPs required the association of apolipoprotein E with lipids but did not require cholesterol. Apoptosis was not prevented by lipids alone or by apoA1- or apoJ-containing lipoproteins. The prevention of neuronal apoptosis was initiated after the binding of LPs to the low-density lipoprotein receptor-related protein (LRP), a multifunctional receptor of the low-density lipoprotein receptor family. We showed that inhibition of LRP activation, by treatment of neurons with receptor-associated protein or anti-LRP antibodies, or by LRP gene-silencing experiments, reduced the protective effect of LPs. Furthermore, another LRP ligand, alpha2-macroglobulin, also protected the neurons from apoptosis. After binding to LRP, LPs initiate a signaling pathway that involves activation of protein kinase Cdelta and inactivation of glycogen synthase kinase-3beta. These findings indicate the potential for using glial lipoproteins or an activator of the LRP signaling pathway for treatment for neurodegenerative disorders such as Alzheimer's disease.
Figures



References
-
- Barres BA, Silverstein BE, Corey DP, Chun LLY. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1:791–803. - PubMed
-
- Bondy CA, Cheng CM. Signaling by insulin-like growth factor 1 in brain. Eur J Pharmacol. 2004;490:25–31. - PubMed
-
- Bright R, Mochly-Rosen D. The role of protein kinase C in cerebral ischemic and reperfusion injury. Stroke. 2005;36:2781–2790. - PubMed
-
- Brown MS, Goldstein JL. Receptor-mediated control of cholesterol metabolism. Science. 1976;191:150–154. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
