Kv4.3-mediated A-type K+ currents underlie rhythmic activity in hippocampal interneurons
- PMID: 17314290
- PMCID: PMC6673559
- DOI: 10.1523/JNEUROSCI.3208-06.2007
Kv4.3-mediated A-type K+ currents underlie rhythmic activity in hippocampal interneurons
Abstract
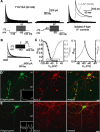
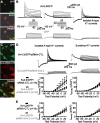
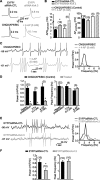
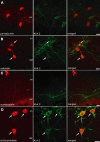
Hippocampal-dependent learning and memory processes are associated with theta frequency rhythmic activity. Interneuron and pyramidal cell network interactions underlie this activity, but contributions of interneuron voltage-gated membrane conductances remain unclear. We show that interneurons at the CA1 lacunosum-moleculare (LM) and radiatum (RAD) junction (LM/RAD) display voltage-dependent subthreshold membrane potential oscillations (MPOs) generated by voltage-gated tetrodotoxin-sensitive Na+ and 4-aminopyridine (4-AP)-sensitive K+ currents. They also exhibit prominent 4-AP-sensitive A-type K+ currents, with gating properties showing activation at subthreshold membrane potentials. We found that LM/RAD cells are part of specific interneuron subpopulations expressing the K+ channel subunit Kv4.3 and their transfection with Kv4.3 small interfering RNA selectively impaired A-type K+ currents and MPOs. Thus, our findings reveal a novel function of Kv4.3-mediated A-type K+ currents in the generation of intrinsic MPOs in specific subpopulations of interneurons that may participate in hippocampal theta-related rhythmic activity.
Figures



References
-
- Alonso A, Llinas RR. Subthreshold Na+-dependent theta-like rhythmicity in stellate cells of entorhinal cortex layer II. Nature. 1989;342:175–177. - PubMed
-
- Alonso A, Llinas RR. Electrophysiology of the mammillary complex in vitro. II. Medial mammillary neurons. J Neurophysiol. 1992;68:1321–1331. - PubMed
-
- Barry DM, Xu H, Schuessler RB, Nerbonne JM. Functional knockout of the transient outward current, long-QT syndrome, and cardiac remodeling in mice expressing a dominant-negative Kv4 alpha subunit. Circ Res. 1998;83:560–567. - PubMed
-
- Birnbaum SG, Varga AW, Yuan LL, Anderson AE, Sweatt JD, Schrader LA. Structure and function of Kv4-family transient potassium channels. Physiol Rev. 2004;84:803–833. - PubMed
-
- Bland BH. The physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol. 1986;26:1–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
