Long-term depression of a dopamine IPSC
- PMID: 17314302
- PMCID: PMC6673562
- DOI: 10.1523/JNEUROSCI.3251-06.2007
Long-term depression of a dopamine IPSC
Abstract
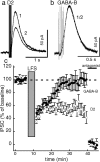
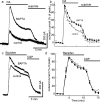
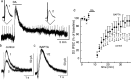
Two determinants of dopamine release from terminals in striatal and limbic structures are the pattern and rate of dopamine neuron firing in the ventral midbrain. This activity is regulated in part by somatodendritic release of dopamine and subsequent feedback inhibition through activation of D2 receptors on dopamine neuron cell bodies and dendrites. This study describes stimulus-dependent long-term depression (LTD) of IPSCs mediated by dopamine. This LTD was blocked by chelation of postsynaptic intracellular calcium, was dependent on the activation of D2 receptors and was independent of glutamate-mediated transmission. Application of a high concentration of dopamine mimicked depression of the IPSC and prevented additional attempts to induce LTD, suggesting that the mechanism of the depression is agonist-dependent receptor activation. Using extracellular recording, there is an inhibition of firing that follows electrical stimulation, and after the induction of LTD the duration of that inhibition was decreased. Reduced inhibition could increase burst firing and action potential-dependent release of dopamine in terminal regions in vivo.
Figures



References
-
- Adell A, Artigas F. The somatodendritic release of dopamine in the ventral tegmental area and its regulation by afferent transmitter systems. Neurosci Biobehav Rev. 2004;28:415–431. - PubMed
-
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. - PubMed
-
- Bellone C, Luscher C. mGluRs induce a long-term depression in the ventral tegmental area that involves a switch of the subunit composition of AMPA receptors. Eur J Neurosci. 2005;21:1280–1288. - PubMed
-
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. - PubMed
-
- Bjorklund A, Lindvall O. Dopamine in dendrites of substantia nigra neurons: suggestions for a role in dendritic terminals. Brain Res. 1975;83:531–537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
