Impaired fertility and spermiogenetic disorders with loss of cell adhesion in male mice expressing an interfering Rap1 mutant
- PMID: 17314400
- PMCID: PMC1838989
- DOI: 10.1091/mbc.e06-10-0902
Impaired fertility and spermiogenetic disorders with loss of cell adhesion in male mice expressing an interfering Rap1 mutant
Abstract
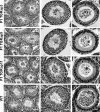
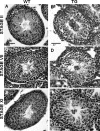
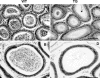
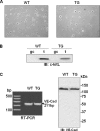
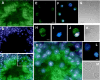
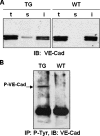
The guanosine trisphosphatase Rap1 serves as a critical player in signal transduction, somatic cell proliferation and differentiation, and cell-cell adhesion by acting through distinct mechanisms. During mouse spermiogenesis, Rap1 is activated and forms a signaling complex with its effector, the serine-threonine kinase B-Raf. To investigate the functional role of Rap1 in male germ cell differentiation, we generated transgenic mice expressing an inactive Rap1 mutant selectively in differentiating spermatids. This expression resulted in a derailment of spermiogenesis due to an anomalous release of immature round spermatids from the seminiferous epithelium within the tubule lumen and in low sperm counts. These spermiogenetic disorders correlated with impaired fertility, with the transgenic males being severely subfertile. Because mutant testis exhibited perturbations in ectoplasmic specializations (ESs), a Sertoli-germ cell-specific adherens junction, we searched for expression of vascular endothelial cadherin (VE-cadherin), an adhesion molecule regulated by Rap1, in spermatogenic cells of wild-type and mutant mice. We found that germ cells express VE-cadherin with a timing strictly related to apical ES formation and function; immature, VE-cadherin-positive spermatids were, however, prematurely released in the transgenic testis. In conclusion, interfering with Rap1 function during spermiogenesis leads to reduced fertility by impairment of germ-Sertoli cell contacts; our transgenic mouse provides an in vivo model to study the regulation of ES dynamics.
Figures





References
-
- Aivatiadou E., Brunetti F., Berruti G. cAMP promotes an Epac2 redistribution where Rap1 is located in differentiating male germ cells. FEBS J. 2005;272(Supplement 1):326.
-
- Andersson A. M., Edvardsen K., Skakkebaek N. E. Expression and localization of N- and E-cadherin in the human testis and epididymis. Int. J. Androl. 1994;17:174–180. - PubMed
-
- Aravindan G. R., Pineau C. P., Bardin C. W., Cheng C. Y. Ability of trypsin in mimicking germ cell factors that affect Sertoli cell secretory function. J. Cell Physiol. 1996;168:123–133. - PubMed
-
- Beardsley A., O'Donnell L. Characterization of normal spermiation and spermiation failure induced by hormone suppression in adult rats. Biol. Reprod. 2003;68:1299–1307. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

