Menstrual blood-derived cells confer human dystrophin expression in the murine model of Duchenne muscular dystrophy via cell fusion and myogenic transdifferentiation
- PMID: 17314403
- PMCID: PMC1855042
- DOI: 10.1091/mbc.e06-09-0872
Menstrual blood-derived cells confer human dystrophin expression in the murine model of Duchenne muscular dystrophy via cell fusion and myogenic transdifferentiation
Abstract
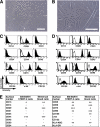
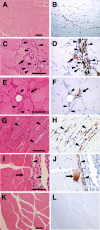
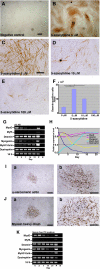
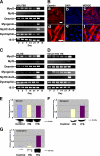
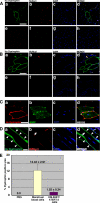
Duchenne muscular dystrophy (DMD), the most common lethal genetic disorder in children, is an X-linked recessive muscle disease characterized by the absence of dystrophin at the sarcolemma of muscle fibers. We examined a putative endometrial progenitor obtained from endometrial tissue samples to determine whether these cells repair muscular degeneration in a murine mdx model of DMD. Implanted cells conferred human dystrophin in degenerated muscle of immunodeficient mdx mice. We then examined menstrual blood-derived cells to determine whether primarily cultured nontransformed cells also repair dystrophied muscle. In vivo transfer of menstrual blood-derived cells into dystrophic muscles of immunodeficient mdx mice restored sarcolemmal expression of dystrophin. Labeling of implanted cells with enhanced green fluorescent protein and differential staining of human and murine nuclei suggest that human dystrophin expression is due to cell fusion between host myocytes and implanted cells. In vitro analysis revealed that endometrial progenitor cells and menstrual blood-derived cells can efficiently transdifferentiate into myoblasts/myocytes, fuse to C2C12 murine myoblasts by in vitro coculturing, and start to express dystrophin after fusion. These results demonstrate that the endometrial progenitor cells and menstrual blood-derived cells can transfer dystrophin into dystrophied myocytes through cell fusion and transdifferentiation in vitro and in vivo.
Figures

References
-
- Aurade F., Pinset C., Chafey P., Gros F., Montarras D. Myf5, MyoD, myogenin and MRF4 myogenic derivatives of the embryonic mesenchymal cell line C3H10T1/2 exhibit the same adult muscle phenotype. Differentiation. 1994;55:185–192. - PubMed
-
- Bischoff R. The satellite cell and muscle regeneration. In: Engel A., Franzini-Armstrong C., Fischman D. A., editors. Myology: Basic and Clinical. New York: McGraw-Hill, Health Professions Division; 1994. pp. 97–118.
-
- Dezawa M., Ishikawa H., Itokazu Y., Yoshihara T., Hoshino M., Takeda S., Ide C., Nabeshima Y. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science. 2005;309:314–317. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

