Scratching the surface of skin development
- PMID: 17314969
- PMCID: PMC2405926
- DOI: 10.1038/nature05659
Scratching the surface of skin development
Abstract
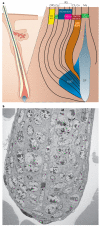
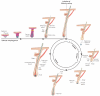
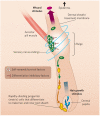
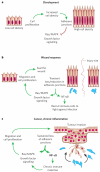
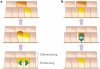
The epidermis and its appendages develop from a single layer of multipotent embryonic progenitor keratinocytes. Embryonic stem cells receive cues from their environment that instruct them to commit to a particular differentiation programme and generate a stratified epidermis, hair follicles or sebaceous glands. Exciting recent developments have focused on how adult skin epithelia maintain populations of stem cells for use in the natural cycles of hair follicle regeneration and for re-epithelialization in response to wounding.
Figures
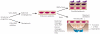
References
-
- Stern CD. Neural induction: old problem, new findings, yet more questions. Development. 2005;132:2007–2021. - PubMed
-
- M'Boneko V, Merker HJ. Development and morphology of the periderm of mouse embryos (days 9–12 of gestation) Acta Anat. (Basel) 1988;133:325–336. - PubMed
-
- Atit R, et al. β-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev. Biol. 2006;296:164–176. - PubMed
-
- Hardy MH. The secret life of the hair follicle. Trends Genet. 1992;8:55–61. - PubMed
-
- Olivera-Martinez I, Thelu J, Dhouailly D. Molecular mechanisms controlling dorsal dermis generation from the somitic dermomyotome. Int. J. Dev. Biol. 2004;48:93–101. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

