Pathophysiology of tumor neovascularization
- PMID: 17315600
- PMCID: PMC1993966
- DOI: 10.2147/vhrm.2005.1.4.277
Pathophysiology of tumor neovascularization
Abstract
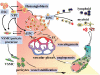
Neovascularization is essential to the process of development and differentiation of tissues in the vertebrate embryo, and is also involved in a wide variety of physiological and pathological conditions in adults, including wound repair, metabolic diseases, inflammation, cardiovascular disorders, and tumor progression. Thanks to cumulative studies on vasculature, new therapeutic approaches have been opened for us to some life-threatening diseases by controlling angiogenesis in the affected organs. In cancer therapy, for example, modulation of factors responsible for tumor angiogenesis may be beneficial in inhibiting of tumor progression. Several antiangiogenic approaches are currently under preclinical trial. However, the mechanisms of neovascularization in tumors are complicated and each tumor shows unique features in its vasculature, depending on tissue specificity, angiogenic micromilieu, grades and stages, host immunity, and so on. For better understanding and effective therapeutic approaches, it is important to clarify both the general mechanism of angiogenic events and the disease-specific mechanism of neovascularization. This review discusses the general features of angiogenesis under physiological and pathological conditions, mainly in tumor progression. In addition, recent topics such as contribution of the endothelial progenitor cells, tumor vasculogenic mimicry, markers for tumor-derived endothelial cells and pericytes, and angiogenic/angiostatic chemokines are summarized.
Figures

References
-
- Adams LD, Geary RL, McManus B, et al. A comparison of aorta and vena cava medial message expression by cDNA array analysis identifies a set of 68 consistently differentially expressed genes, all in aortic media. Circ Res. 2000;87:623–31. - PubMed
-
- Aicher A, Brenner W, Zuhayra M, et al. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation. 2003;107:2134–9. - PubMed
-
- Alavi A, Hood JD, Frausto R, et al. Role of Raf in vascular protection from distinct apoptotic stimuli. Science. 2003;301:94–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

