Dynorphin peptides differentially regulate the human kappa opioid receptor
- PMID: 17316701
- PMCID: PMC2696490
- DOI: 10.1016/j.lfs.2007.01.018
Dynorphin peptides differentially regulate the human kappa opioid receptor
Abstract
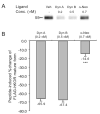
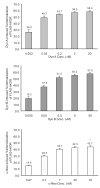
Dynorphins, endogenous peptides for the kappa opioid receptor, play important roles in many physiological and pathological functions. Here, we examined how prolonged treatment with three major prodynorphin peptides, dynorphin A (1-17) (Dyn A), dynorphin B (1-13) (Dyn B) and alpha-neoendorphin (alpha-Neo), regulated the human kappa opioid receptor (hKOR) stably expressed in Chinese hamster ovary (CHO) cells. Results from receptor binding and [(35)S]GTPgammaS binding assays showed that these peptides were potent full agonists of the hKOR with comparable receptor reserve and intrinsic efficacy to stimulate G proteins. A 4-h incubation with alpha-Neo at a concentration of approximately 600xEC(50) value (from [(35)S]GTPgammaS binding) resulted in receptor down-regulation to a much lower extent than the incubation with Dyn A and Dyn B at comparable concentrations ( approximately 10% vs. approximately 65%). Extending incubation period and increasing concentrations did not significantly affect the difference. The plateau level of alpha-Neo-mediated receptor internalization (30 min) was significantly less than those of Dyn A and Dyn B. Omission of the serum from the incubation medium or addition of peptidase inhibitors into the serum-containing medium enhanced alpha-Neo-, but not Dyn A- or Dyn B-, mediated receptor down-regulation and internalization; however, the degrees of alpha-Neo-induced adaptations were still significantly less than those of Dyn A and Dyn B. Thus, these endogenous peptides differentially regulate KOR after activating the receptor with similar receptor occupancy and intrinsic efficacy. Both stability in the presence of serum and intrinsic capacity to promote receptor adaptation play roles in the observed discrepancy among the dynorphin peptides.
Figures



References
-
- Akil H, Owens C, Gutstein H, Taylor L, Curran E, Watson S. Endogenous opioids: overview and current issues. Drug & Alcohol Dependence. 1998;51(12):127–140. - PubMed
-
- Arden JR, Segredo V, Wang Z, Lameh J, Sadee W. Phosphorylation and agonist-specific intracellular trafficking of an epitope-tagged mu-opioid receptor expressed in HEK 293 cells. Journal of Neurochemistry. 1995;65:1636–1645. - PubMed
-
- Bot G, Blake AD, Li S, Reisine T. Opioid regulation of the mouse delta-opioid receptor expressed in human embryonic kidney 293 cells. Molecular Pharmacology. 1997;52(2):272–281. - PubMed
-
- Checler F. Peptidases and neuropeptide-inactivating mechanisms in the circulation and in the gastrointestinal tract. In: Daniel EE, editor. Neuropeptide Function in the Gastrointestinal Tract. CRC Press; Boca Raton, FL: 1991. pp. 273–308.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

