Induction of immunity to neuroblastoma early after syngeneic hematopoietic stem cell transplantation using a novel mouse tumor vaccine
- PMID: 17317581
- PMCID: PMC1852542
- DOI: 10.1016/j.bbmt.2006.11.018
Induction of immunity to neuroblastoma early after syngeneic hematopoietic stem cell transplantation using a novel mouse tumor vaccine
Abstract
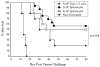
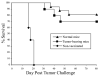
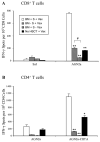
Autologous HSCT has resulted in improved event-free survival in patients with advanced neuroblastoma, but most of these patients still relapse. We previously reported that transient transfection of mouse neuroblastoma cells with plasmid DNA vectors encoding immune costimulatory molecules generates cell-based vaccines capable of inducing potent antitumor T cell immunity. In this study, we explored the effectiveness of tumor vaccine administration soon after HSCT. Soon after transplantation, only vaccinated mice that had received an adoptive transfer of syngeneic T cells survived tumor challenge. Tumor protective immunity in the transplant recipients was dependent on CD4(+) and CD8(+) T cells, and tumor-reactive T cells in the spleens of vaccinated mice could be detected in IFN-gamma enzyme-linked immunosorbent spot (ELISPOT) assays. Our data indicate that the adoptive transfer of T cells was absolutely required for induction of protective immunity by the tumor vaccine. Adoptive transfer of T cells accelerated T cell reconstitution, but it also resulted in increased percentages of CD4(+)CD25(+)Foxp3(+) cells soon after HSCT. Treatment of HSC transplant recipients with an anti-CD25 mAb before tumor vaccination inhibited antitumor immunity and significantly decreased the number of IFN-gamma-secreting tumor-specific CD4 T cells. However, physical depletion of CD25(+) cells from the adoptively transferred splenocytes appeared to increase the efficacy of tumor vaccination. Collectively, these results demonstrate that anti-neuroblastoma immunity can be induced soon after HSCT using a novel cell-based cancer vaccine. However, sufficient numbers of T cells must be added to the graft to achieve protective antitumor immunity, and depletion of CD25(+) T cells from adoptively transferred T cells might provide some additional benefit. These translational studies will aid in our development of post-HSCT vaccines for neuroblastoma.
Figures



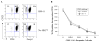


Similar articles
-
Depletion of CD25⁺ T cells from hematopoietic stem cell grafts increases posttransplantation vaccine-induced immunity to neuroblastoma.Blood. 2011 Jun 23;117(25):6952-62. doi: 10.1182/blood-2010-12-326108. Epub 2011 Apr 26. Blood. 2011. PMID: 21521781 Free PMC article.
-
Depletion of CD4 T cells enhances immunotherapy for neuroblastoma after syngeneic HSCT but compromises development of antitumor immune memory.Blood. 2009 Apr 30;113(18):4449-57. doi: 10.1182/blood-2008-11-190827. Epub 2009 Jan 30. Blood. 2009. PMID: 19182203 Free PMC article.
-
CD25+ regulatory T cell inhibition enhances vaccine-induced immunity to neuroblastoma.J Immunother. 2007 Feb-Mar;30(2):203-14. doi: 10.1097/01.cji.0000211336.91513.dd. J Immunother. 2007. PMID: 17471167
-
Graft Engineering and Adoptive Immunotherapy: New Approaches to Promote Immune Tolerance After Hematopoietic Stem Cell Transplantation.Front Immunol. 2019 Jul 10;10:1342. doi: 10.3389/fimmu.2019.01342. eCollection 2019. Front Immunol. 2019. PMID: 31354695 Free PMC article. Review.
-
Adoptive cellular therapy.Curr Top Microbiol Immunol. 2011;344:149-72. doi: 10.1007/82_2010_94. Curr Top Microbiol Immunol. 2011. PMID: 20700700 Review.
Cited by
-
Examining T cells at vaccine sites of tumor-bearing hosts provides insights to dysfunctional T-cell immunity.J Immunother. 2013 Jan;36(1):41-51. doi: 10.1097/CJI.0b013e318274590e. J Immunother. 2013. PMID: 23211619 Free PMC article.
-
Early expression of stem cell-associated genes within the CD8 compartment after treatment with a tumor vaccine.Cell Immunol. 2010;265(1):65-73. doi: 10.1016/j.cellimm.2010.07.004. Epub 2010 Jul 17. Cell Immunol. 2010. PMID: 20692654 Free PMC article.
-
Depletion of CD25⁺ T cells from hematopoietic stem cell grafts increases posttransplantation vaccine-induced immunity to neuroblastoma.Blood. 2011 Jun 23;117(25):6952-62. doi: 10.1182/blood-2010-12-326108. Epub 2011 Apr 26. Blood. 2011. PMID: 21521781 Free PMC article.
-
Early immunisation with dendritic cells after allogeneic bone marrow transplantation elicits graft vs tumour reactivity.Br J Cancer. 2013 Mar 19;108(5):1092-9. doi: 10.1038/bjc.2013.39. Br J Cancer. 2013. PMID: 23511628 Free PMC article.
-
Immunomodulation with dendritic cells and donor lymphocyte infusion converge to induce graft vs neuroblastoma reactions without GVHD after allogeneic bone marrow transplantation.Br J Cancer. 2010 Nov 9;103(10):1597-605. doi: 10.1038/sj.bjc.6605924. Epub 2010 Oct 26. Br J Cancer. 2010. PMID: 20978501 Free PMC article.
References
-
- Carachi R. Perspectives on neuroblastoma. Pediatr Surg Int. 2002;18:299–305. - PubMed
-
- Sarkar AK, Nuchtern JG. Lysis of MYCN-amplified neuroblastoma cells by MYCN peptide-specific cytotoxic T lymphocytes. Cancer Res. 2000;60:1908–13. - PubMed
-
- Rodolfo M, Luksch R, Stockert E, et al. Antigen-specific immunity in neuroblastoma patients: antibody and T-cell recognition of NY-ESO-1 tumor antigen. Cancer Res. 2003;63:6948–55. - PubMed
-
- Oberthuer A, Hero B, Spitz R, Berthold F, Fischer M. The tumor-associated antigen PRAME is universally expressed in high-stage neuroblastoma and associated with poor outcome. Clin Cancer Res. 2004;10:4307–13. - PubMed
-
- Johnson BD, Yan X, Schauer DW, Orentas RJ. Dual expression of CD80 and CD86 produces a tumor vaccine superior to single expression of either molecule. Cell Immunol. 2003;222:15–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

