Investigation of thin filament near-neighbour regulatory unit interactions during force development in skinned cardiac and skeletal muscle
- PMID: 17317743
- PMCID: PMC2075566
- DOI: 10.1113/jphysiol.2007.128975
Investigation of thin filament near-neighbour regulatory unit interactions during force development in skinned cardiac and skeletal muscle
Abstract
Ca(2+)-dependent activation of striated muscle involves cooperative interactions of cross-bridges and thin filament regulatory proteins. We investigated how interactions between individual structural regulatory units (RUs; 1 tropomyosin, 1 troponin, 7 actins) influence the level and rate of demembranated (skinned) cardiac muscle force development by exchanging native cardiac troponin (cTn) with different ratio mixtures of wild-type (WT) cTn and cTn containing WT cardiac troponin T/I + cardiac troponin C (cTnC) D65A (a site II inactive cTnC mutant). Maximal Ca(2+)-activated force (F(max)) increased in less than a linear manner with WT cTn. This contrasts with results we obtained previously in skeletal fibres (using sTnC D28A, D65A) where F(max) increased in a greater than linear manner with WT sTnC, and suggests that Ca(2+) binding to each functional Tn activates < 7 actins of a structural regulatory unit in cardiac muscle and > 7 actins in skeletal muscle. The Ca(2+) sensitivity of force and rate of force redevelopment (k(tr)) was leftward shifted by 0.1-0.2 -log [Ca(2+)] (pCa) units as WT cTn content was increased, but the slope of the force-pCa relation and maximal k(tr) were unaffected by loss of near-neighbour RU interactions. Cross-bridge inhibition (with butanedione monoxime) or augmentation (with 2 deoxy-ATP) had no greater effect in cardiac muscle with disruption of near-neighbour RU interactions, in contrast to skeletal muscle fibres where the effect was enhanced. The rate of Ca(2+) dissociation was found to be > 2-fold faster from whole cardiac Tn compared with skeletal Tn. Together the data suggest that in cardiac (as opposed to skeletal) muscle, Ca(2+) binding to individual Tn complexes is insufficient to completely activate their corresponding RUs, making thin filament activation level more dependent on concomitant Ca(2+) binding at neighbouring Tn sites and/or crossbridge feedback effects on Ca(2+) binding affinity.
Figures

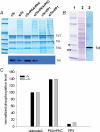



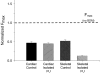

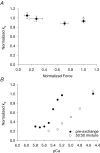
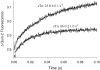
Comment in
-
Local control in thin filament activation of cardiac muscle.J Physiol. 2007 Apr 15;580(Pt. 2):358. doi: 10.1113/jphysiol.2007.131672. Epub 2007 Mar 8. J Physiol. 2007. PMID: 17347259 Free PMC article. No abstract available.
Similar articles
-
Thin-filament regulation of force redevelopment kinetics in rabbit skeletal muscle fibres.J Physiol. 2007 Mar 1;579(Pt 2):313-26. doi: 10.1113/jphysiol.2006.124164. Epub 2007 Jan 4. J Physiol. 2007. PMID: 17204497 Free PMC article.
-
Influence of enhanced troponin C Ca2+-binding affinity on cooperative thin filament activation in rabbit skeletal muscle.J Physiol. 2007 Aug 15;583(Pt 1):337-50. doi: 10.1113/jphysiol.2007.135426. Epub 2007 Jun 21. J Physiol. 2007. PMID: 17584846 Free PMC article.
-
Thin filament near-neighbour regulatory unit interactions affect rabbit skeletal muscle steady-state force-Ca(2+) relations.J Physiol. 2002 Apr 15;540(Pt 2):485-97. doi: 10.1113/jphysiol.2001.013179. J Physiol. 2002. PMID: 11956338 Free PMC article.
-
Regulation of contraction in striated muscle.Physiol Rev. 2000 Apr;80(2):853-924. doi: 10.1152/physrev.2000.80.2.853. Physiol Rev. 2000. PMID: 10747208 Review.
-
Focus on cardiac troponin complex: From gene expression to cardiomyopathy.Genes Dis. 2024 Mar 11;11(6):101263. doi: 10.1016/j.gendis.2024.101263. eCollection 2024 Nov. Genes Dis. 2024. PMID: 39211905 Free PMC article. Review.
Cited by
-
Slowed Dynamics of Thin Filament Regulatory Units Reduces Ca2+-Sensitivity of Cardiac Biomechanical Function.Cell Mol Bioeng. 2013 Jun 1;6(2):183-198. doi: 10.1007/s12195-013-0269-8. Cell Mol Bioeng. 2013. PMID: 23833690 Free PMC article.
-
Contributions of Ca2+-Independent Thin Filament Activation to Cardiac Muscle Function.Biophys J. 2015 Nov 17;109(10):2101-12. doi: 10.1016/j.bpj.2015.09.028. Biophys J. 2015. PMID: 26588569 Free PMC article.
-
A Stochastic Multiscale Model of Cardiac Thin Filament Activation Using Brownian-Langevin Dynamics.Biophys J. 2019 Dec 17;117(12):2255-2272. doi: 10.1016/j.bpj.2019.08.003. Epub 2019 Aug 9. Biophys J. 2019. PMID: 31547973 Free PMC article.
-
Structural basis for the in situ Ca(2+) sensitization of cardiac troponin C by positive feedback from force-generating myosin cross-bridges.Arch Biochem Biophys. 2013 Sep 15;537(2):198-209. doi: 10.1016/j.abb.2013.07.013. Epub 2013 Jul 26. Arch Biochem Biophys. 2013. PMID: 23896515 Free PMC article.
-
Calcium has a direct effect on thick filament activation in porcine myocardium.J Gen Physiol. 2024 Nov 4;156(11):e202413545. doi: 10.1085/jgp.202413545. Epub 2024 Sep 20. J Gen Physiol. 2024. PMID: 39302315 Free PMC article.
References
-
- Brenner B. The cross-bridge cycle in muscle. Mechanical, biochemical, and structural studies on single skinned rabbit psoas fibers to characterize cross-bridge kinetics in muscle for correlation with the actomyosin-ATPase in solution. Basic Res Cardiol. 1986;81(Suppl. 1):1–15. - PubMed
-
- Butters CA, Tobacman JB, Tobacman LS. Cooperative effect of Ca2+ binding to adjacent troponin molecules on the thin filament-myosin subfragment 1 MgATPase rate. J Biol Chem. 1997;272:13196–13202. - PubMed
-
- Chandy IK, Lo JC, Ludescher RD. Differential mobility of skeletal and cardiac tropomyosin on the surface of F-actin. Biochemistry. 1999;38:9286–9294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

