The role of synaptotagmin I C2A calcium-binding domain in synaptic vesicle clustering during synapse formation
- PMID: 17317745
- PMCID: PMC2075219
- DOI: 10.1113/jphysiol.2006.127472
The role of synaptotagmin I C2A calcium-binding domain in synaptic vesicle clustering during synapse formation
Abstract
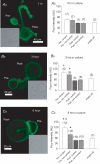
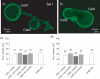
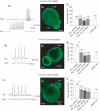
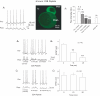
Synaptic vesicles aggregate at the presynaptic terminal during synapse formation via mechanisms that are poorly understood. Here we have investigated the role of the putative calcium sensor synaptotagmin I in vesicle aggregation during the formation of soma-soma synapses between identified partner cells using a simple in vitro synapse model in the mollusc Lymnaea stagnalis. Immunocytochemistry, optical imaging and electrophysiological recording techniques were used to monitor synapse formation and vesicle localization. Within 6 h, contact between appropriate synaptic partner cells up-regulated global synaptotagmin I expression, and induced a localized aggregation of synaptotagmin I at the contact site. Cell contacts between non-synaptic partner cells did not affect synaptotagmin I expression. Application of an human immunodeficiency virus type-1 transactivator (HIV-1 TAT)-tagged peptide corresponding to loop 3 of the synaptotagmin I C2A domain prevented synaptic vesicle aggregation and synapse formation. By contrast, a TAT-tagged peptide containing the calcium-binding motif of the C2B domain did not affect synaptic vesicle aggregation or synapse formation. Calcium imaging with Fura-2 demonstrated that TAT-C2 peptides did not alter either basal or evoked intracellular calcium levels. These results demonstrate that contact with an appropriate target cell is necessary to initiate synaptic vesicle aggregation during nascent synapse formation and that the initial aggregation of synaptic vesicles is dependent on loop 3 of the C2A domain of synaptotagmin I.
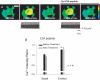
Figures



References
-
- Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci. 2000;3:445–451. - PubMed
-
- Antz C, Geyer M, Fakler B, Schott MK, Guy HR, Frank R, Ruppersberg JP, Kalbitzer HR. NMR structure of inactivation gates from mammalian voltage-dependent potassium channels. Nature. 1997;385:272–275. - PubMed
-
- Atwood HL, Karunanithi S. Diversification of synaptic strength: presynaptic elements. Nat Rev Neurosci. 2002;3:497–516. - PubMed
-
- Augustine GJ. How does calcium trigger neurotransmitter release? Curr Opin Neurobiol. 2001;11:320–326. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

