Differential effects of cyclic and constant stress on ATP release and mucociliary transport by human airway epithelia
- PMID: 17317749
- PMCID: PMC2075559
- DOI: 10.1113/jphysiol.2006.126086
Differential effects of cyclic and constant stress on ATP release and mucociliary transport by human airway epithelia
Abstract
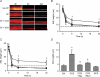
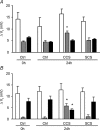
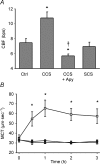
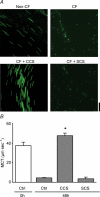
In the lungs, the first line of defence against bacterial infection is the thin layer of airway surface liquid (ASL) lining the airway surface. The superficial airway epithelium exhibits complex regulatory pathways that blend ion transport to adjust ASL volume to maintain proper mucociliary clearance (MCC). We hypothesized that stresses generated by airflow and transmural pressures during breathing govern ASL volume by regulating the rate of epithelial ATP release. Luminal ATP, via interactions with apical membrane P2-purinoceptors, regulates the balance of active ion secretion versus absorption to maintain ASL volume at optimal levels for MCC. In this study we tested the hypothesis that cyclic compressive stress (CCS), mimicking normal tidal breathing, regulates ASL volume in airway epithelia. Polarized tracheobronchial epithelial cultures from normal and cystic fibrosis (CF) subjects responded to a range of CCS by increasing the rate of ATP release. In normal airway epithelia, the CCS-induced increase in ASL ATP concentration was sufficient to induce purinoceptor-mediated increases in ASL height and MCC, via inhibition of epithelial Na(+)-channel-mediated Na(+) absorption and stimulation of Cl(-) secretion through CFTR and the Ca(2+)-activated chloride channels. In contrast, static, non-oscillatory stress did not stimulate ATP release, ion transport or MCC, emphasizing the importance of rhythmic mechanical stress for airway defence. In CF airway cultures, which exhibit basal ASL depletion, CCS was partially effective, producing less ASL volume secretion than in normal cultures, but a level sufficient to restore MCC. The present data suggest that CCS may (1) regulate ASL volume in the normal lung and (2) improve clearance in the lungs of CF patients, potentially explaining the beneficial role of exercise in lung defence.
Figures
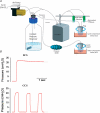
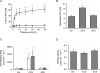
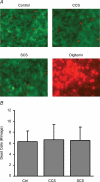
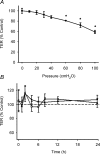
Comment in
-
Extracellular autocrine nucleotide signalling in a microenvironment: integrative physiology in a minute volume of airway surface liquid.J Physiol. 2007 Apr 15;580(Pt. 2):359-60. doi: 10.1113/jphysiol.2007.131805. Epub 2007 Mar 15. J Physiol. 2007. PMID: 17363380 Free PMC article. No abstract available.
References
-
- Basser PJ, McMahon TA, Griffith P. The mechanism of mucus clearance in cough. J Biomech Eng. 1989;111:288–297. - PubMed
-
- Benali R, Pierrot D, Zahm JM, de Bentzmann S, Puchelle E. Effect of extracellular ATP and UTP on fluid transport by human nasal epithelial cells in culture. Am J Respir Cell Mol Biol. 1994;10:363–368. - PubMed
-
- Boucher RC. Human airway ion transport. Part two. Am J Respir Crit Care Med. 1994;150:581–593. - PubMed
-
- Boucher RC. An overview of the pathogenesis of cystic fibrosis lung disease. Adv Drug Deliv Rev. 2002;54:1359–1371. - PubMed
-
- Boucher RC. Regulation of airway surface liquid volume by human airway epithelia. Pflugers Arch. 2003;445:495–498. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

