Beta-catenin stabilization stalls the transition from double-positive to single-positive stage and predisposes thymocytes to malignant transformation
- PMID: 17317856
- PMCID: PMC1890819
- DOI: 10.1182/blood-2006-11-059071
Beta-catenin stabilization stalls the transition from double-positive to single-positive stage and predisposes thymocytes to malignant transformation
Abstract
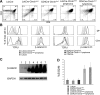
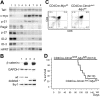
Activation of beta-catenin has been causatively linked to the etiology of colon cancer. Conditional stabilization of this molecule in pro-T cells promotes thymocyte development without the requirement for pre-TCR signaling. We show here that activated beta-catenin stalls the developmental transition from the double-positive (DP) to the single-positive (SP) thymocyte stage and predisposes DP thymocytes to transformation. beta-Catenin-induced thymic lymphomas have a leukemic arrest at the early DP stage. Lymphomagenesis requires Rag activity, which peaks at this developmental stage, as well as additional secondary genetic events. A consistent secondary event is the transcriptional up-regulation of c-Myc, whose activity is required for transformation because its conditional ablation abrogates lymphomagenesis. In contrast, the expression of Notch receptors as well as targets is reduced in DP thymocytes with stabilized beta-catenin and remains low in the lymphomas, indicating that Notch activation is not required or selected for in beta-catenin-induced lymphomas. Thus, beta-catenin activation may provide a mechanism for the induction of T-cell-acute lymphoblastic leukemia (T-ALL) that does not depend on Notch activation.
Figures





References
-
- Behrens J, Jerchow BA, Wurtele M, et al. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–599. - PubMed
-
- Kishida S, Yamamoto H, Ikeda S, et al. Axin, a negative regulator of the wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of beta-catenin. J Biol Chem. 1998;273:10823–10826. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

