Cdt1 associates dynamically with chromatin throughout G1 and recruits Geminin onto chromatin
- PMID: 17318181
- PMCID: PMC1817642
- DOI: 10.1038/sj.emboj.7601597
Cdt1 associates dynamically with chromatin throughout G1 and recruits Geminin onto chromatin
Abstract
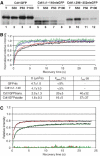
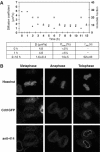
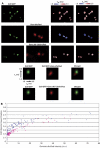
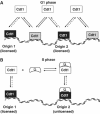
To maintain genome integrity, eukaryotic cells initiate DNA replication once per cell cycle after assembling prereplicative complexes (preRCs) on chromatin at the end of mitosis and during G1. In S phase, preRCs are disassembled, precluding initiation of another round of replication. Cdt1 is a key member of the preRC and its correct regulation via proteolysis and by its inhibitor Geminin is essential to prevent premature re-replication. Using quantitative fluorescence microscopy, we study the interactions of Cdt1 with chromatin and Geminin in living cells. We find that Cdt1 exhibits dynamic interactions with chromatin throughout G1 phase and that the protein domains responsible for chromatin and Geminin interactions are separable. Contrary to existing in vitro data, we show that Cdt1 simultaneously binds Geminin and chromatin in vivo, thereby recruiting Geminin onto chromatin. We propose that dynamic Cdt1-chromatin associations and the recruitment of Geminin to chromatin provide spatio-temporal control of the licensing process.
Figures



Similar articles
-
Cdt1 interactions in the licensing process: a model for dynamic spatiotemporal control of licensing.Cell Cycle. 2007 Jul 1;6(13):1549-52. doi: 10.4161/cc.6.13.4455. Epub 2007 May 22. Cell Cycle. 2007. PMID: 17598984 Review.
-
Inhibition of eukaryotic DNA replication by geminin binding to Cdt1.Science. 2000 Dec 22;290(5500):2309-12. doi: 10.1126/science.290.5500.2309. Science. 2000. PMID: 11125146
-
A Cdt1-geminin complex licenses chromatin for DNA replication and prevents rereplication during S phase in Xenopus.EMBO J. 2006 Dec 13;25(24):5764-74. doi: 10.1038/sj.emboj.7601436. Epub 2006 Nov 23. EMBO J. 2006. PMID: 17124498 Free PMC article.
-
Cell cycle regulation of the licensing activity of Cdt1 in Xenopus laevis.Exp Cell Res. 2004 Apr 15;295(1):138-49. doi: 10.1016/j.yexcr.2003.11.018. Exp Cell Res. 2004. PMID: 15051497
-
Geminin-Cdt1 balance is critical for genetic stability.Mutat Res. 2005 Jan 6;569(1-2):111-21. doi: 10.1016/j.mrfmmm.2004.05.026. Mutat Res. 2005. PMID: 15603756 Review.
Cited by
-
Proliferating cell nuclear antigen-dependent rapid recruitment of Cdt1 and CRL4Cdt2 at DNA-damaged sites after UV irradiation in HeLa cells.J Biol Chem. 2010 Dec 31;285(53):41993-2000. doi: 10.1074/jbc.M110.161661. Epub 2010 Oct 7. J Biol Chem. 2010. PMID: 20929861 Free PMC article.
-
The contribution of dormant origins to genome stability: from cell biology to human genetics.DNA Repair (Amst). 2014 Jul;19(100):182-9. doi: 10.1016/j.dnarep.2014.03.012. Epub 2014 Apr 24. DNA Repair (Amst). 2014. PMID: 24767947 Free PMC article. Review.
-
HN1 Is Enriched in the S-Phase, Phosphorylated in Mitosis, and Contributes to Cyclin B1 Degradation in Prostate Cancer Cells.Biology (Basel). 2023 Jan 26;12(2):189. doi: 10.3390/biology12020189. Biology (Basel). 2023. PMID: 36829467 Free PMC article.
-
Ring of Change: CDC48/p97 Drives Protein Dynamics at Chromatin.Front Genet. 2016 May 3;7:73. doi: 10.3389/fgene.2016.00073. eCollection 2016. Front Genet. 2016. PMID: 27200082 Free PMC article. Review.
-
Idas, a novel phylogenetically conserved geminin-related protein, binds to geminin and is required for cell cycle progression.J Biol Chem. 2011 Jul 1;286(26):23234-46. doi: 10.1074/jbc.M110.207688. Epub 2011 May 4. J Biol Chem. 2011. PMID: 21543332 Free PMC article.
References
-
- Aparicio OM, Weinstein DM, Bell SP (1997) Components and dynamics of DNA replication complexes in S. cerevisiae: redistribution of MCM proteins and Cdc45p during S phase. Cell 91: 59–69 - PubMed
-
- Arias EE, Walter JC (2006) PCNA functions as a molecular platform to trigger Cdt1 destruction and prevent re-replication. Nat Cell Biol 8: 84–90 - PubMed
-
- Bastiaens PI, Squire A (1999) Fluorescence lifetime imaging microscopy: spatial resolution of biochemical processes in the cell. Trends Cell Biol 9: 48–52 - PubMed
-
- Bell SP, Dutta A (2002) DNA replication in eukaryotic cells. Annu Rev Biochem 71: 333–374 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

