Ubiquitination of the GTPase Rap1B by the ubiquitin ligase Smurf2 is required for the establishment of neuronal polarity
- PMID: 17318188
- PMCID: PMC1817628
- DOI: 10.1038/sj.emboj.7601580
Ubiquitination of the GTPase Rap1B by the ubiquitin ligase Smurf2 is required for the establishment of neuronal polarity
Retraction in
-
Retraction: 'Ubiquitination of the GTPase Rap1B by the ubiquitin ligase Smurf2 is required for the establishment of neuronal polarity'.EMBO J. 2014 Dec 17;33(24):3012. doi: 10.15252/embj.201470130. EMBO J. 2014. PMID: 25520303 Free PMC article. No abstract available.
Abstract
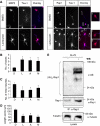
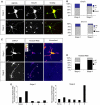
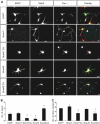
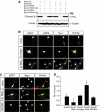
The development of a polarised morphology with multiple dendrites and a single axon is an essential step in the differentiation of neurons. The establishment of neuronal polarity is directed by the sequential activity of the GTPases Rap1B and Cdc42. Rap1B is initially present in all neurites of unpolarised neurons, but becomes restricted to the tip of a single process during the establishment of neuronal polarity where it specifies axonal identity. Here, we show that the ubiquitin ligases Smad ubiquitination regulatory factor-1 (Smurf1) and Smurf2 are essential for neurite growth and neuronal polarity, respectively, and regulate the GTPases Rho and Rap1B in hippocampal neurons. Smurf2 is required for the restriction of Rap1B to a single neurite. Smurf2 ubiquitinates inactive Rap1B and initiates its degradation through the ubiquitin/proteasome pathway (UPS). Degradation of Rap1B restricts it to a single neurite and thereby ensures that neurons extend a single axon.
Figures

Similar articles
-
The interaction of mPar3 with the ubiquitin ligase Smurf2 is required for the establishment of neuronal polarity.J Biol Chem. 2007 Nov 30;282(48):35259-68. doi: 10.1074/jbc.M703438200. Epub 2007 Sep 28. J Biol Chem. 2007. Retraction in: J Biol Chem. 2015 Jun 19;290(25):15391. doi: 10.1074/jbc.A115.703438. PMID: 17906294 Retracted.
-
Rheb and mTOR regulate neuronal polarity through Rap1B.J Biol Chem. 2008 Nov 28;283(48):33784-92. doi: 10.1074/jbc.M802431200. Epub 2008 Oct 8. J Biol Chem. 2008. PMID: 18842593 Free PMC article.
-
The sequential activity of the GTPases Rap1B and Cdc42 determines neuronal polarity.Nat Neurosci. 2004 Sep;7(9):923-9. doi: 10.1038/nn1295. Epub 2004 Aug 1. Nat Neurosci. 2004. PMID: 15286792
-
Establishment and plasticity of neuronal polarity.J Neurosci Res. 1999 Sep 1;57(5):577-89. J Neurosci Res. 1999. PMID: 10462683 Review.
-
Regulation of neuronal/axonal degeneration by ZNRF1 ubiquitin ligase.Neurosci Res. 2019 Feb;139:21-25. doi: 10.1016/j.neures.2018.07.008. Epub 2018 Aug 15. Neurosci Res. 2019. PMID: 30118738 Review.
Cited by
-
Exchange Protein Directly Activated by cAMP (EPAC) Regulates Neuronal Polarization through Rap1B.J Neurosci. 2015 Aug 12;35(32):11315-29. doi: 10.1523/JNEUROSCI.3645-14.2015. J Neurosci. 2015. PMID: 26269639 Free PMC article.
-
miR-9 and miR-124 synergistically affect regulation of dendritic branching via the AKT/GSK3β pathway by targeting Rap2a.Sci Rep. 2016 May 25;6:26781. doi: 10.1038/srep26781. Sci Rep. 2016. PMID: 27221778 Free PMC article.
-
A tumor suppressor function of Smurf2 associated with controlling chromatin landscape and genome stability through RNF20.Nat Med. 2012 Jan 8;18(2):227-34. doi: 10.1038/nm.2596. Nat Med. 2012. PMID: 22231558 Free PMC article.
-
Galanin stimulates neurite outgrowth from sensory neurons by inhibition of Cdc42 and Rho GTPases and activation of cofilin.J Neurochem. 2013 Oct;127(2):199-208. doi: 10.1111/jnc.12379. Epub 2013 Aug 22. J Neurochem. 2013. PMID: 23895321 Free PMC article.
-
Smurf2 regulates the senescence response and suppresses tumorigenesis in mice.Cancer Res. 2012 Jun 1;72(11):2714-9. doi: 10.1158/0008-5472.CAN-11-3773. Epub 2012 May 2. Cancer Res. 2012. PMID: 22552287 Free PMC article.
References
-
- Bos JL, de Rooij J, Reedquist KA (2001) Rap1 signalling: adhering to new models. Nat Rev Mol Cell Biol 2: 369–377 - PubMed
-
- Bradke F, Dotti CG (1997) Neuronal polarity: vectorial cytoplasmic flow precedes axon formation. Neuron 19: 1175–1186 - PubMed
-
- Bradke F, Dotti CG (2000) Establishment of neuronal polarity: lessons from cultured hippocampal neurons. Curr Opin Neurobiol 10: 574–581 - PubMed
-
- Campbell DS, Holt CE (2001) Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron 32: 1013–1026 - PubMed
-
- Campbell DS, Holt CE (2003) Apoptotic pathway and MAPKs differentially regulate chemotropic responses of retinal growth cones. Neuron 37: 939–952 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

