Bcr-Abl stabilizes beta-catenin in chronic myeloid leukemia through its tyrosine phosphorylation
- PMID: 17318191
- PMCID: PMC1817619
- DOI: 10.1038/sj.emboj.7601485
Bcr-Abl stabilizes beta-catenin in chronic myeloid leukemia through its tyrosine phosphorylation
Abstract
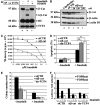
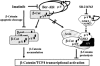
Self-renewal of Bcr-Abl(+) chronic myeloid leukemia (CML) cells is sustained by a nuclear activated serine/threonine-(S/T) unphosphorylated beta-catenin. Although beta-catenin can be tyrosine (Y)-phosphorylated, the occurrence and biological relevance of this covalent modification in Bcr-Abl-associated leukemogenesis is unknown. Here we show that Bcr-Abl levels control the degree of beta-catenin protein stabilization by affecting its Y/S/T-phospho content in CML cells. Bcr-Abl physically interacts with beta-catenin, and its oncogenic tyrosine kinase activity is required to phosphorylate beta-catenin at Y86 and Y654 residues. This Y-phospho beta-catenin binds to the TCF4 transcription factor, thus representing a transcriptionally active pool. Imatinib, a Bcr-Abl antagonist, impairs the beta-catenin/TCF-related transcription causing a rapid cytosolic retention of Y-unphosphorylated beta-catenin, which presents an increased binding affinity for the Axin/GSK3beta complex. Although Bcr-Abl does not affect GSK3beta autophosphorylation, it prevents, through its effect on beta-catenin Y phosphorylation, Axin/GSK3beta binding to beta-catenin and its subsequent S/T phosphorylation. Silencing of beta-catenin by small interfering RNA inhibited proliferation and clonogenicity of Bcr-Abl(+) CML cells, in synergism with Imatinib. These findings indicate the Bcr-Abl triggered Y phosphorylation of beta-catenin as a new mechanism responsible for its protein stabilization and nuclear signalling activation in CML.
Figures






References
-
- Barnes DJ, Palaiologou D, Panousopoulou E, Schultheis B, Yong A, Wong A, Pattacini L, Goldman JM, Melo JV (2005) Bcr-Abl expression levels determinate the rate of development of resistance to Imatinib mesylate in chronic myeloid leukaemia. Cancer Res 65: 8912–8919 - PubMed
-
- Coluccia AM, Benati D, Dekhil H, De Filippo A, Lan C, Gambacorti Passerini C (2006) SKI-606 decreases growth and motility of colorectal cancer cells by preventing pp60(c-Src)-dependent tyrosine phosphorylation of β-catenin and its nuclear signalling. Cancer Res 66: 2279–2286 - PubMed
-
- Daley GQ (2004) Chronic myeloid leukemia: proving ground for cancer stem cells. Cell 119: 314–316 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

