Matrix metalloproteinases and the regulation of tissue remodelling
- PMID: 17318226
- PMCID: PMC2760082
- DOI: 10.1038/nrm2125
Matrix metalloproteinases and the regulation of tissue remodelling
Abstract
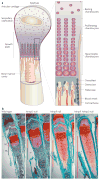
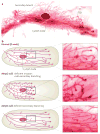
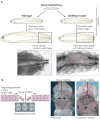
Matrix metalloproteinases (MMPs) were discovered because of their role in amphibian metamorphosis, yet they have attracted more attention because of their roles in disease. Despite intensive scrutiny in vitro, in cell culture and in animal models, the normal physiological roles of these extracellular proteases have been elusive. Recent studies in mice and flies point to essential roles of MMPs as mediators of change and physical adaptation in tissues, whether developmentally regulated, environmentally induced or disease associated.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nature Rev Mol Cell Biol. 2002;3:207–214. - PubMed
-
- Birkedal-Hansen H, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. - PubMed
-
- Welgus HG, Kobayashi DK, Jeffrey JJ. The collagen substrate specificity of rat uterus collagenase. J Biol Chem. 1983;258:14162–14165. - PubMed
-
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nature Rev Cancer. 2002;2:161–174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

