Impaired neutrophil activity and increased susceptibility to bacterial infection in mice lacking glucose-6-phosphatase-beta
- PMID: 17318259
- PMCID: PMC1797608
- DOI: 10.1172/JCI30443
Impaired neutrophil activity and increased susceptibility to bacterial infection in mice lacking glucose-6-phosphatase-beta
Abstract
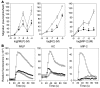
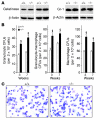
Neutropenia and neutrophil dysfunction are common in many diseases, although their etiology is often unclear. Previous views held that there was a single ER enzyme, glucose-6-phosphatase-alpha (G6Pase-alpha), whose activity--limited to the liver, kidney, and intestine--was solely responsible for the final stages of gluconeogenesis and glycogenolysis, in which glucose-6-phosphate (G6P) is hydrolyzed to glucose for release to the blood. Recently, we characterized a second G6Pase activity, that of G6Pase-beta (also known as G6PC), which is also capable of hydrolyzing G6P to glucose but is ubiquitously expressed and not implicated in interprandial blood glucose homeostasis. We now report that the absence of G6Pase-beta led to neutropenia; defects in neutrophil respiratory burst, chemotaxis, and calcium flux; and increased susceptibility to bacterial infection. Consistent with this, G6Pase-beta-deficient (G6pc3-/-) mice with experimental peritonitis exhibited increased expression of the glucose-regulated proteins upregulated during ER stress in their neutrophils and bone marrow, and the G6pc3-/- neutrophils exhibited an enhanced rate of apoptosis. Our results define a molecular pathway to neutropenia and neutrophil dysfunction of previously unknown etiology, providing a potential model for the treatment of these conditions.
Figures
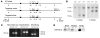


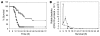


References
-
- Chou J.Y., Matern D., Mansfield B.C., Chen Y.T. Type I glycogen storage diseases: disorders of the glucose-6-phosphatase complex. Curr. Mol. Med. 2002;2:121–143. - PubMed
-
- Chou, J.Y., and Mansfield, B.C. 2003. Glucose-6-phosphate transporter: the key to glycogen storage disease type Ib. In Membrane transporter diseases. S. Broer and C.A. Wagner, editors. Springer. New York, New York, USA. 191–205.
-
- Beaudet A.L., Anderson D.C., Michels V.V., Arion W.J., Lange A.J. Neutropenia and impaired neutrophil migration in type 1B glycogen storage disease. J. Pediatr. 1980;97:906–910. - PubMed
-
- Gitzelmann R., Bosshard N.U. Defective neutrophil and monocyte functions in glycogen storage disease type 1b: a literature review. Eur. J. Pediatr. 1993;152(Suppl.):S33–S38. - PubMed
-
- Rothbaum R., et al. Shwachman-Diamond syndrome: report from an international conference. J. Pediatr. 2002;141:266–270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

