Dimerization between vasopressin V1b and corticotropin releasing hormone type 1 receptors
- PMID: 17318384
- PMCID: PMC11517283
- DOI: 10.1007/s10571-006-9135-8
Dimerization between vasopressin V1b and corticotropin releasing hormone type 1 receptors
Abstract
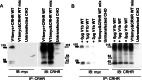
1. Increasing evidence indicates that guanyl protein coupled receptors (GPCRs), including members of the vasopressin (VP) receptor family can act as homo- and heterodimers. Regulated expression and interaction of pituitary VP V1b receptor (V1bR) and corticotropin releasing hormone receptor type 1 (CRHR1) are critical for hypothalamic pituitary adrenal (HPA) axis adaptation, but it is unknown whether this involves physical interaction between these receptors.2. Bioluminescence resonance energy transfer (BRET) experiments using V1bR and CRHR1 fused to either Renilla luciferase (Rluc) or yellow fluorescent protein (YFP) at the N-terminus, but not the carboxyl-terminus, revealed specific interaction (BRET(50) = 0.39 +/- 0.08, V1bR) that was inhibited by untagged V1b or CRHR1 receptors, suggesting homo- and heterodimerization. The BRET data were confirmed by coimmunoprecipitation experiments using fully bioactive receptors tagged at the aminoterminus with c-myc and Flag epitopes, demonstrating specific homodimerization of the V1b receptor and heterodimerization of the V1b receptor with CRHR1 receptors.3. Heterodimerization between V1bR and CRHR1 is not ligand dependent since stimulation with CRH and AVP had no effect on coimmunoprecipitation. In membranes obtained from cells cotransfected with CRHR1 and V1bR, incubation with the heterologous nonpeptide antagonist did not alter the binding affinity or capacity of the receptor.4. The data demonstrate that V1bR and CRHR1 can form constitutive homo- and heterodimers and suggests that the heterodimerization does not influence the binding properties of these receptors.
Figures






Similar articles
-
V1b and CRHR1 receptor heterodimerization mediates synergistic biological actions of vasopressin and CRH.Mol Endocrinol. 2012 Mar;26(3):502-20. doi: 10.1210/me.2011-1202. Epub 2012 Feb 2. Mol Endocrinol. 2012. PMID: 22301784 Free PMC article.
-
Characterization of a functional V1B vasopressin receptor in the male rat kidney: evidence for cross talk between V1B and V2 receptor signaling pathways.Am J Physiol Renal Physiol. 2021 Sep 1;321(3):F305-F321. doi: 10.1152/ajprenal.00081.2021. Epub 2021 Jul 20. Am J Physiol Renal Physiol. 2021. PMID: 34282956
-
Acute and chronic regulation of pituitary receptors for vasopressin and corticotropin releasing hormone.Arch Physiol Biochem. 2002 Apr;110(1-2):74-89. doi: 10.1076/apab.110.1.74.905. Arch Physiol Biochem. 2002. PMID: 11935403 Review.
-
The vasopressin V1b receptor critically regulates hypothalamic-pituitary-adrenal axis activity under both stress and resting conditions.J Clin Invest. 2004 Jan;113(2):302-9. doi: 10.1172/JCI19656. J Clin Invest. 2004. PMID: 14722621 Free PMC article.
-
Vasopressinergic regulation of the hypothalamic pituitary adrenal axis and stress adaptation.Stress. 2004 Jun;7(2):75-83. doi: 10.1080/10253890410001733535. Stress. 2004. PMID: 15512850 Review.
Cited by
-
Receptor oligomerization in family B1 of G-protein-coupled receptors: focus on BRET investigations and the link between GPCR oligomerization and binding cooperativity.Front Endocrinol (Lausanne). 2012 May 7;3:62. doi: 10.3389/fendo.2012.00062. eCollection 2012. Front Endocrinol (Lausanne). 2012. PMID: 22649424 Free PMC article.
-
Copeptin as a marker of an altered CRH axis in pituitary disease.Endocrine. 2017 Sep;57(3):474-480. doi: 10.1007/s12020-017-1366-6. Epub 2017 Aug 9. Endocrine. 2017. PMID: 28795329 Free PMC article.
-
Response of substances co-expressed in hypothalamic magnocellular neurons to osmotic challenges in normal and Brattleboro rats.Cell Mol Neurobiol. 2008 Dec;28(8):1033-47. doi: 10.1007/s10571-008-9306-x. Epub 2008 Sep 5. Cell Mol Neurobiol. 2008. PMID: 18773290 Free PMC article. Review.
-
Post-synaptic density-95 (PSD-95) binding capacity of G-protein-coupled receptor 30 (GPR30), an estrogen receptor that can be identified in hippocampal dendritic spines.J Biol Chem. 2013 Mar 1;288(9):6438-50. doi: 10.1074/jbc.M112.412478. Epub 2013 Jan 8. J Biol Chem. 2013. PMID: 23300088 Free PMC article.
-
Emerging role of alternative splicing of CRF1 receptor in CRF signaling.Acta Biochim Pol. 2010;57(1):1-13. Epub 2010 Mar 16. Acta Biochim Pol. 2010. PMID: 20234885 Free PMC article. Review.
References
-
- AbdAlla, S., Lother, H., and Quitterer, U. (2000). AT1-receptor heterodimers show enhanced G-protein activation and altered receptor sequestration. Nature407:94–98. - PubMed
-
- Abou-Samra, A. B., Harwood, J. P., Manganiello, V. C., Catt, K. J., and Aguilera, G. (1987). Phorbol 12-myristate 13-acetate and vasopressin potentiate the effect of corticotropin-releasing factor on cyclic AMP production in rat anterior pituitary cells: Mechanisms of action. J. Biol. Chem.262:1129–1136. - PubMed
-
- Aguilera, G. (1994). Regulation of pituitary ACTH secretion during chronic stress. Front. Neuroendocrinol.15:321–350. - PubMed
-
- Aguilera, G., Pham, Q., and Rabadan-Diehl, C. (1994). Regulation of pituitary vasopressin receptors during chronic stress: Relationship to corticotroph responsiveness. J. Neuroendocrinol.6:299–304. - PubMed
-
- Ayoub, M. A., Couturier, C., Lucas-Meunier, E., Angers, S., Fossier, P., Bouvier, M., and Jockers, R. (2002). Monitoring of ligand-independent dimerization and ligand-induced conformational changes of melatonin receptors in living cells by bioluminescence resonance energy transfer. J. Biol. Chem.277:21522–21528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

