Insulin glargine in the treatment of type 1 and type 2 diabetes
- PMID: 17319470
- PMCID: PMC1993975
- DOI: 10.2147/vhrm.2006.2.1.59
Insulin glargine in the treatment of type 1 and type 2 diabetes
Abstract
Insulin glargine is an analogue of human insulin that is modified to provide a consistent level of plasma insulin over a long duration. Pharmacokinetic and pharmacodynamic studies show that a single injection of insulin glargine leads to a smooth 24-hour time-action profile with no undesirable pronounced peaks of activity. In clinical trials, this profile has been associated with at least equivalent, if not better, glycemic control than other traditional basal insulins and a significantly lower rate of overall and nocturnal hypoglycemia. The convenience of a once-daily injection, a lack of need for resuspension (insulin glargine is a clear solution when injected), and lower rates of hypoglycemia should translate into improvements in patient treatment satisfaction. This review appraises the evidence for the view that insulin glargine represents an advance in basal insulin therapy for both type 1 and type 2 diabetes patients.
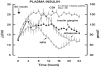
Figures

Similar articles
-
Switching to multiple daily injection therapy with glulisine improves glycaemic control, vascular damage and treatment satisfaction in basal insulin glargine-injected diabetic patients.Diabetes Metab Res Rev. 2014 Nov;30(8):693-700. doi: 10.1002/dmrr.2537. Diabetes Metab Res Rev. 2014. PMID: 24639403 Clinical Trial.
-
An update on the treatment of type 1 and type 2 diabetes mellitus: focus on insulin detemir, a long-acting human insulin analog.Vasc Health Risk Manag. 2010 Jun 1;6:399-410. doi: 10.2147/vhrm.s10397. Vasc Health Risk Manag. 2010. PMID: 20539842 Free PMC article. Review.
-
Negative binomial meta-regression analysis of combined glycosylated hemoglobin and hypoglycemia outcomes across eleven Phase III and IV studies of insulin glargine compared with neutral protamine Hagedorn insulin in type 1 and type 2 diabetes mellitus.Clin Ther. 2007 Aug;29(8):1607-19. doi: 10.1016/j.clinthera.2007.08.020. Clin Ther. 2007. PMID: 17919543
-
Insulin glargine: commentary on the duration of action and lower risk of nocturnal hypoglycaemia in patients with diabetes.Expert Opin Pharmacother. 2004 Jan;5(1):1-2; author reply 2-3. doi: 10.1517/14656566.5.1.1. Expert Opin Pharmacother. 2004. PMID: 14680430 No abstract available.
-
Insulin glargine: a review of its therapeutic use as a long-acting agent for the management of type 1 and 2 diabetes mellitus.Drugs. 2001;61(11):1599-624. doi: 10.2165/00003495-200161110-00007. Drugs. 2001. PMID: 11577797 Review.
Cited by
-
Effectiveness and Safety of Adding Basal Insulin Glargine in Patients with Type 2 Diabetes Mellitus Exhibiting Inadequate Response to Metformin and DPP-4 Inhibitors with or without Sulfonylurea.Diabetes Metab J. 2019 Aug;43(4):432-446. doi: 10.4093/dmj.2018.0092. Epub 2019 Jun 19. Diabetes Metab J. 2019. PMID: 31237133 Free PMC article. Clinical Trial.
-
Impact of switching from neutral protamine hagedorn insulin (NPH) to glargine insulin on glycemic control and clinical outcomes in pediatric patients with type 1 diabetes.Explor Res Clin Soc Pharm. 2025 May 2;18:100612. doi: 10.1016/j.rcsop.2025.100612. eCollection 2025 Jun. Explor Res Clin Soc Pharm. 2025. PMID: 40491723 Free PMC article.
-
The Affordable Care Act and Diabetes Diagnosis and Care: Exploring the Potential Impacts.Curr Diab Rep. 2016 Apr;16(4):27. doi: 10.1007/s11892-016-0712-z. Curr Diab Rep. 2016. PMID: 26892908 Free PMC article. Review.
-
Hypoglycemic episodes in hospitalized people with diabetes in Portugal: the HIPOS-WARD study.Clin Diabetes Endocrinol. 2021 Jan 5;7(1):2. doi: 10.1186/s40842-020-00114-3. Clin Diabetes Endocrinol. 2021. PMID: 33402217 Free PMC article.
-
Effects of exercise training combined with insulin treatment on cardiac NOS1 signaling pathways in type 1 diabetic rats.Mol Cell Biochem. 2011 Jan;347(1-2):53-62. doi: 10.1007/s11010-010-0611-6. Epub 2010 Oct 9. Mol Cell Biochem. 2011. PMID: 20936328
References
-
- Bahr M, Kolter T, Seipke G, et al. Growth promoting and metabolic activity of the human insulin analogue [GlyA21, ArgB31, ArgB32] insulin (HOE 901) in muscle cells. Eur J Pharmacol. 1997;320:259–65. - PubMed
-
- Berti L, Kellerer M, Bossenmaier B, et al. The long acting human insulin analog HOE 901: characteristics of insulin signalling in comparison to Asp(B10) and regular insulin. Horm Metab Res. 1998;30:123–9. - PubMed
-
- Bohannon NJV. Optimizing insulin regimens in type 1 diabetes. How to help patients get control of their life. Postgraduate Medicine. Postgrad Med. 2003;113:39–42. 45–8, 54. - PubMed
-
- Bolli GB, Owens DR. Insulin glargine. Lancet. 2000;356:443–5. - PubMed
-
- Campbell RK, White JR., Jr Insulin therapy in type 2 diabetes. J Am Pharm Assoc (Wash) 2002;42:602–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

