Cardiolipin switch in mitochondria: shutting off the reduction of cytochrome c and turning on the peroxidase activity
- PMID: 17319652
- PMCID: PMC3356783
- DOI: 10.1021/bi061854k
Cardiolipin switch in mitochondria: shutting off the reduction of cytochrome c and turning on the peroxidase activity
Abstract
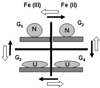
Upon interaction with anionic phospholipids, particularly mitochondria-specific cardiolipin (CL), cytochrome c (cyt c) loses its tertiary structure and its peroxidase activity dramatically increases. CL-induced peroxidase activity of cyt c has been found to be important for selective CL oxidation in cells undergoing programmed death. During apoptosis, the peroxidase activity and the fraction of CL-bound cyt c markedly increase, suggesting that CL may act as a switch to regulate cyt c's mitochondrial functions. Using cyclic voltammetry and equilibrium redox titrations, we show that the redox potential of cyt c shifts negatively by 350-400 mV upon binding to CL-containing membranes. Consequently, functions of cyt c as an electron transporter and cyt c reduction by Complex III are strongly inhibited. Further, CL/cyt c complexes are not effective in scavenging superoxide anions and are not effectively reduced by ascorbate. Thus, both redox properties and functions of cyt c change upon interaction with CL in the mitochondrial membrane, diminishing cyt c's electron donor/acceptor role and stimulating its peroxidase activity.
Figures




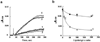




References
-
- Mathews CK, van Holde KE, Ahem KG. Biochemistry. third ed. San Francisco: Addison Wesley Longman; 1999.
-
- Pettigrew GW, Moore GR. Cytochrome c - Biological aspects. Berlin, Heidelberg: Springer-Verlaf; 1987.
-
- Pereverzev MO, Vygodina TV, Konstantinov AA, Skulachev VP. Cytochrome c an ideal antioxidant. Biochem Soc Trans. 2003;31:1312–1315. - PubMed
-
- Gogvadze V, Orrenius S, Zhivotovsky B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim Biophys Acta. 2006;1757:639–647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

