Efficient ligation of the Schistosoma hammerhead ribozyme
- PMID: 17319693
- PMCID: PMC3203546
- DOI: 10.1021/bi062077r
Efficient ligation of the Schistosoma hammerhead ribozyme
Abstract
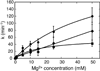
The hammerhead ribozyme from Schistosoma mansoni is the best characterized of the natural hammerhead ribozymes. Biophysical, biochemical, and structural studies have shown that the formation of the loop-loop tertiary interaction between stems I and II alters the global folding, cleavage kinetics, and conformation of the catalytic core of this hammerhead, leading to a ribozyme that is readily cleaved under physiological conditions. This study investigates the ligation kinetics and the internal equilibrium between cleavage and ligation for the Schistosoma hammerhead. Single turnover kinetic studies on a construct where the ribozyme cleaves and ligates substrate(s) in trans showed up to 23% ligation when starting from fully cleaved products. This was achieved by an approximately 2000-fold increase in the rate of ligation compared to a minimal hammerhead without the loop-loop tertiary interaction, yielding an internal equilibrium that ranges from 2 to 3 at physiological Mg2+ ion concentrations (0.1-1 mM). Thus, the natural Schistosoma hammerhead ribozyme is almost as efficient at ligation as it is at cleavage. The results here are consistent with a model where formation of the loop-loop tertiary interaction leads to a higher population of catalytically active molecules and where formation of this tertiary interaction has a much larger effect on the ligation than the cleavage activity of the Schistosoma hammerhead ribozyme.
Figures






Similar articles
-
Characterization of a native hammerhead ribozyme derived from schistosomes.RNA. 2005 Feb;11(2):187-96. doi: 10.1261/rna.7950605. RNA. 2005. PMID: 15659358 Free PMC article.
-
Fast cleavage kinetics of a natural hammerhead ribozyme.J Am Chem Soc. 2004 Sep 8;126(35):10848-9. doi: 10.1021/ja046848v. J Am Chem Soc. 2004. PMID: 15339162
-
Two Divalent Metal Ions and Conformational Changes Play Roles in the Hammerhead Ribozyme Cleavage Reaction.Biochemistry. 2015 Oct 20;54(41):6369-81. doi: 10.1021/acs.biochem.5b00824. Epub 2015 Oct 2. Biochemistry. 2015. PMID: 26398724 Free PMC article.
-
Hammerhead redux: does the new structure fit the old biochemical data?RNA. 2008 Apr;14(4):605-15. doi: 10.1261/rna.912608. Epub 2008 Feb 20. RNA. 2008. PMID: 18287565 Free PMC article. Review.
-
Structural Simplicity and Mechanistic Complexity in the Hammerhead Ribozyme.Prog Mol Biol Transl Sci. 2018;159:177-202. doi: 10.1016/bs.pmbts.2018.07.006. Epub 2018 Sep 17. Prog Mol Biol Transl Sci. 2018. PMID: 30340787 Review.
Cited by
-
RNA Back and Forth: Looking through Ribozyme and Viroid Motifs.Viruses. 2019 Mar 21;11(3):283. doi: 10.3390/v11030283. Viruses. 2019. PMID: 30901893 Free PMC article.
-
Hammerhead Ribozymes: Structural Insights, Catalytic Mechanisms, and Cutting-Edge Applications in Synthetic Biology.Int J Mol Sci. 2025 Jun 12;26(12):5624. doi: 10.3390/ijms26125624. Int J Mol Sci. 2025. PMID: 40565088 Free PMC article. Review.
-
Enhanced product stability in the hammerhead ribozyme.Biochemistry. 2010 Jun 1;49(21):4494-500. doi: 10.1021/bi902025m. Biochemistry. 2010. PMID: 20423112 Free PMC article.
-
Ligation of RNA Oligomers by the Schistosoma mansoni Hammerhead Ribozyme in Frozen Solution.J Mol Evol. 2016 Mar;82(2-3):81-92. doi: 10.1007/s00239-016-9729-9. Epub 2016 Feb 20. J Mol Evol. 2016. PMID: 26897022
-
Structure-based search reveals hammerhead ribozymes in the human microbiome.J Biol Chem. 2011 Mar 11;286(10):7737-7743. doi: 10.1074/jbc.C110.209288. Epub 2011 Jan 21. J Biol Chem. 2011. PMID: 21257745 Free PMC article.
References
-
- Steitz TA, Moore PB. RNA, the first macromolecular catalyst: the ribosome is a ribozyme. Trends Biochem. Sci. 2003;28:411–418. - PubMed
-
- Bashan A, Agmon I, Zarivach R, Schluenzen F, Harms J, Berisio R, Bartels H, Franceschi F, Auerbach T, Hansen HAS, Kossoy E, Kessler M, Yonath A. Structural basis of the ribosomal machinery for peptide bond formation, translocation, and nascent chain progression. Mol. Cell. 2003;11:91–102. - PubMed
-
- Kruger K, Grabowski PJ, Zaug AJ, Sands J, Gottschling DE, Cech TR. Self-splicing RNA - Auto-excision and auto-cyclization of the ribosomal-RNA intervening sequence of Tetrahymena. Cell. 1982;31:147–157. - PubMed
-
- Peebles CL, Perlman PS, Mecklenburg KL, Petrillo ML, Tabor JH, Jarrell KA, Cheng HL. A self-splicing RNA excises an intron lariat. Cell. 1986;44:213–223. - PubMed
-
- Vanderveen R, Arnberg AC, Vanderhorst G, Bonen L, Tabak HF, Grivell LA. Excised Group-II introns in Yeast mitochondria are lariats and can be formed by self-splicing invitro. Cell. 1986;44:225–234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

