Design of a retrospective patient record study on the occurrence of adverse events among patients in Dutch hospitals
- PMID: 17319971
- PMCID: PMC1810530
- DOI: 10.1186/1472-6963-7-27
Design of a retrospective patient record study on the occurrence of adverse events among patients in Dutch hospitals
Abstract
Background: Various international studies have shown that a substantial number of patients suffer from injuries or even die as a result of care delivered in hospitals. The occurrence of injuries among patients caused by health care management in Dutch hospitals has never been studied systematically. Therefore, an epidemiological study was initiated to determine the incidence, type and impact of adverse events among discharged and deceased patients in Dutch hospitals.
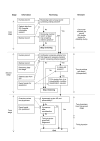
Methods/design: Three stage retrospective patient record review study in 21 hospitals of 8400 patient records of discharged or deceased patients in 2004. The records were reviewed by trained nurses and physicians between August 2005 and October 2006. In addition to the determination of presence, the degree of preventability, and causes of adverse events, also location, timing, classification, and most responsible specialty of the adverse events were measured. Moreover, patient and admission characteristics and the quality of the patient records were recorded.
Discussion: In this paper we report on the design of the retrospective patient record study on the occurrence of adverse events in Dutch hospitals. Attention is paid to the strengths and limitations of the study design. Furthermore, alterations made in the original research protocol in comparison with former international studies are described in detail.
Figures
References
-
- Baker GR, Norton PG, Flintoft V, Blais R, Brown A, Cox J, Etchells E, Ghali WA, Hebert P, Majumdar SR, O' Beirne M, Palacios-Derflingher L, Reid RJ, Sheps S, Tamblyn R. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ. 2004;170:1678–1686. - PMC - PubMed
-
- Brennan TA, Leape LL, Laird NM, Hebert L, Localio AR, Lawthers AG, Newhouse JP, Weiler PC, Hiatt HH. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324:370–376. - PubMed
-
- Davis P, Lay-Yee R, Briant R, Ali W, Scott A, Schug S. Adverse events in New Zealand public hospitals I: occurrence and impact. N Z Med J. 2002;115:U271. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical