Extracellular glutamine is a critical modulator for regulatory volume increase in human glioma cells
- PMID: 17320059
- PMCID: PMC1899165
- DOI: 10.1016/j.brainres.2007.01.085
Extracellular glutamine is a critical modulator for regulatory volume increase in human glioma cells
Abstract
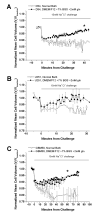
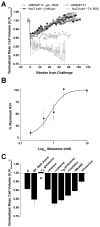
Mammalian cells regulate their volume to prevent unintentional changes in intracellular signaling, cell metabolism, and DNA integrity. Intentional cell volume changes occur as cells undergo proliferation, apoptosis, or cell migration. To regulate cell volume, cells use channels and transport systems to flux osmolytes across the plasma membrane followed by the obligatory movement of water. While essentially all cells are capable of regulatory volume decrease (RVD), regulatory volume increase (RVI) mechanisms have only been reported in some cell types. In this investigation, we used human glioma cells as a model system to determine conditions necessary for RVI. When exposed to hyperosmotic conditions through the addition of 30 mosM NaCl or sucrose, D54-MG and U251 glioma cell lines and glioma cells from acute patient biopsies shrunk transiently but were able to fully recover their original cell volume within 40-70 min. This ability was highly temperature sensitive and absolutely required the presence of low millimolar concentrations of l-glutamine in the extracellular solution. Other known substrates of glutamine transporters such as methyl-amino isobutyric acid (MeAIB), alanine, and threonine were unable to support RVI. The ability of cells to undergo RVI also required the presence of Na+, K+, and Cl- and was inhibited by the NKCC inhibitor, bumetanide, consistent with the involvement of a Na+/K+/2Cl- cotransporter (NKCC). Moreover, the expression of NKCC1 was demonstrated by Western blot. We concluded that regulatory volume increase in human glioma cells occurs through the uptake of Na+, K+, and Cl- by NKCC1 and is modulated by the presence of glutamine.
Figures
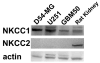
Similar articles
-
Inhibition of Na(+)-K(+)-2Cl(-) cotransporter isoform 1 accelerates temozolomide-mediated apoptosis in glioblastoma cancer cells.Cell Physiol Biochem. 2012;30(1):33-48. doi: 10.1159/000339047. Epub 2012 Jun 8. Cell Physiol Biochem. 2012. PMID: 22759954 Free PMC article.
-
Volume regulation in mammalian skeletal muscle: the role of sodium-potassium-chloride cotransporters during exposure to hypertonic solutions.J Physiol. 2011 Jun 1;589(Pt 11):2887-99. doi: 10.1113/jphysiol.2011.206730. Epub 2011 Apr 11. J Physiol. 2011. PMID: 21486779 Free PMC article.
-
Regulatory volume increase in epithelial cells isolated from the mouse fourth ventricle choroid plexus involves Na(+)-H(+) exchange but not Na(+)-K(+)-2Cl(-) cotransport.Brain Res. 2010 Apr 6;1323:1-10. doi: 10.1016/j.brainres.2009.12.094. Epub 2010 Feb 7. Brain Res. 2010. PMID: 20144884
-
Role of WNK Kinases in the Modulation of Cell Volume.Curr Top Membr. 2018;81:207-235. doi: 10.1016/bs.ctm.2018.08.002. Epub 2018 Aug 29. Curr Top Membr. 2018. PMID: 30243433 Review.
-
The bumetanide-sensitive Na-K-2Cl cotransporter NKCC1 as a potential target of a novel mechanism-based treatment strategy for neonatal seizures.Neurosurg Focus. 2008 Sep;25(3):E22. doi: 10.3171/FOC/2008/25/9/E22. Neurosurg Focus. 2008. PMID: 18759624 Review.
Cited by
-
With-No-Lysine Kinase 3 (WNK3) stimulates glioma invasion by regulating cell volume.Am J Physiol Cell Physiol. 2011 Nov;301(5):C1150-60. doi: 10.1152/ajpcell.00203.2011. Epub 2011 Aug 3. Am J Physiol Cell Physiol. 2011. PMID: 21813709 Free PMC article.
-
Ammonia, like K(+), stimulates the Na(+), K(+), 2 Cl(-) cotransporter NKCC1 and the Na(+),K(+)-ATPase and interacts with endogenous ouabain in astrocytes.Neurochem Res. 2015 Feb;40(2):241-57. doi: 10.1007/s11064-014-1352-9. Epub 2014 Jun 15. Neurochem Res. 2015. PMID: 24929663 Review.
-
Cortical GABAergic excitation contributes to epileptic activities around human glioma.Sci Transl Med. 2014 Jul 9;6(244):244ra89. doi: 10.1126/scitranslmed.3008065. Sci Transl Med. 2014. PMID: 25009229 Free PMC article.
-
alpha-ENaC is a functional element of the hypertonicity-induced cation channel in HepG2 cells and it mediates proliferation.Pflugers Arch. 2009 Aug;458(4):675-87. doi: 10.1007/s00424-009-0649-z. Epub 2009 Feb 25. Pflugers Arch. 2009. PMID: 19241091 Free PMC article.
-
Ion Channels in Glioma Malignancy.Rev Physiol Biochem Pharmacol. 2021;181:223-267. doi: 10.1007/112_2020_44. Rev Physiol Biochem Pharmacol. 2021. PMID: 32930879 Review.
References
-
- Broer S, Brookes N. Transfer of glutamine between astrocytes and neurons. J Neurochem. 2001;77:705–719. - PubMed
-
- Bussolati O, Uggeri J, Belletti S, Dall’Asta V, Gazzola GC. The stimulation of Na, K, Cl cotransport and of system A for neutral amino acid transport is a mechanism for cell volume increase during the cell cycle. FASEB J. 1996;10:920–926. - PubMed
-
- Chassande O, Frelin C, Farahifar D, Jean T, Lazdunski M. The Na+/K+/Cl− cotransport in C6 glioma cells. Properties and role in volume regulation. Eur J Biochem. 1988;171:425–433. - PubMed
-
- Dall’Asta V, Rossi PA, Bussolati O, Gazzola GC. Response of human fibroblasts to hypertonic stress. Cell shrinkage is counteracted by an enhanced active transport of neutral amino acids. J Biol Chem. 1994;269:10485–10491. - PubMed
-
- Dmitrieva NI, Burg MB. Hypertonic stress response. Mutat Res. 2005;569:65–74. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

