A focused salivary gland infection with attenuated MCMV: an animal model with prevention of pathology associated with systemic MCMV infection
- PMID: 17320076
- PMCID: PMC3506192
- DOI: 10.1016/j.yexmp.2006.12.010
A focused salivary gland infection with attenuated MCMV: an animal model with prevention of pathology associated with systemic MCMV infection
Abstract
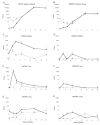
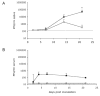
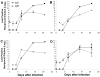
While the salivary gland has been recognized as an important effector site of the common mucosal immune system, a useful model for studying anti-viral salivary gland immune responses in vivo and for exploring the role of the salivary gland within the common mucosal system has been lacking. Murine cytomegalovirus (MCMV) is a beta-herpesvirus that displays a strong tropism for the salivary gland and produces significant morbidity in susceptible mice when introduced by intraperitoneal (i.p.) inoculation. This study tested the hypothesis that MCMV morbidity and pathology could be reduced by injecting the virus directly the submandibular salivary gland (intraglandular (i.g.)), using either in vivo derived MCMV or the less virulent, tissue-culture-derived MCMV (tcMCMV). Peak salivary gland viral titers were completely unaffected by infection route (i.p vs. i.g.) after inoculation with either MCMV or tcMCMV. However, i.g. tcMCMV inoculation reduced viremia in all systemic tissues tested compared to i.p. inoculation. Furthermore, systemic organ pathology observed in the liver and spleen after i.p. inoculation with either MCMV or tcMCMV was completely eliminated by i.g. inoculation with tcMCMV. Cellular infiltrates in the salivary glands, after i.p. or i.g. inoculation were composed of both B and T cells, indicating the potential for a local immune response to occur in the salivary gland. These results demonstrate that a focused MCMV infection of the salivary gland without systemic organ pathology is possible using i.g. delivery of tcMCMV.
Figures


References
-
- Allan JE, Shellam GR. Genetic control of murine cytomegalovirus infection: virus titers in resistant and susceptible strains of mice. Arch Virol. 1984;81(1–2):139–150. - PubMed
-
- Bergmeier LA, Tao L, Gearing AJM, Adams S, Lehner T. Induction of IgA and IgG antibodies in vaginal fluid, serum and saliva following immunization of genital and gut associated lymphoid tissue. In: Mestecky J, editor. Advances in Mucosal Immunology. Plenum Press; New York: 1995. pp. 1567–1573. - PubMed
-
- Britt WJ, Alford CA. Cytomegalovirus. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. Lippincott-Raven; Philadelphia: 1996. pp. 2493–2523.
-
- Brody AR, Craighead JE. Pathogenesis of pulmonary cytomegalovirus infection in immunosuppressed mice. J Infect Dis. 1974;129(6):677–89. - PubMed
-
- Castellano G, Hazzard, Madden DL, Sever JL. Comparison of the enzyme-linked immunosorbent assay and the indirect hemagglutination test for detection of antibody to cytomegalovirus. J, Infect, Dis. 1977;136:S337–S340. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

