The post-translational phenotype of collagen synthesized by SAOS-2 osteosarcoma cells
- PMID: 17320498
- PMCID: PMC1909750
- DOI: 10.1016/j.bone.2007.01.011
The post-translational phenotype of collagen synthesized by SAOS-2 osteosarcoma cells
Abstract
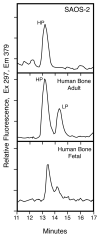
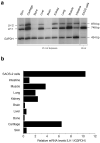
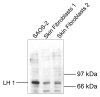
The human osteosarcoma-derived cell line, SAOS-2, exhibits many of the phenotypic characteristics of osteoblasts including the deposition of types I and V collagens in an extracellular matrix. Lesser amounts of collagen XI chains were also detected. The cell layer collagen contains hydroxylysyl pyridinoline cross-links but without the accompanying lysyl pyridinoline typical of human bone collagen. This indicates that the lysine residues at the two helical cross-linking loci are fully hydroxylated. The isoform of lysyl hydroxylase, LH1, known to be required for full hydroxylation at these sites, was shown to be highly expressed by SAOS-2 cells. Our findings provide insight on the mechanism of post-translational overmodification of lysine residues in collagen made by osteosarcoma tumors, and may be relevant for understanding a similar overmodification observed in osteoporotic bone.
Figures





Similar articles
-
Lysyl hydroxylase-2b directs collagen cross-linking pathways in MC3T3-E1 cells.J Bone Miner Res. 2004 Aug;19(8):1349-55. doi: 10.1359/JBMR.040323. Epub 2004 Mar 29. J Bone Miner Res. 2004. PMID: 15231023
-
Effect of hyper- and microgravity on collagen post-translational controls of MC3T3-E1 osteoblasts.J Bone Miner Res. 2003 Sep;18(9):1695-705. doi: 10.1359/jbmr.2003.18.9.1695. J Bone Miner Res. 2003. PMID: 12968680
-
Disentangling mechanisms involved in collagen pyridinoline cross-linking: The immunophilin FKBP65 is critical for dimerization of lysyl hydroxylase 2.Proc Natl Acad Sci U S A. 2016 Jun 28;113(26):7142-7. doi: 10.1073/pnas.1600074113. Epub 2016 Jun 13. Proc Natl Acad Sci U S A. 2016. PMID: 27298363 Free PMC article.
-
Prolyl and lysyl hydroxylases in collagen synthesis.Exp Dermatol. 2021 Jan;30(1):38-49. doi: 10.1111/exd.14197. Epub 2020 Oct 15. Exp Dermatol. 2021. PMID: 32969070 Review.
-
Collagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevance.Bone. 1998 Mar;22(3):181-7. doi: 10.1016/s8756-3282(97)00279-2. Bone. 1998. PMID: 9514209 Review.
Cited by
-
Cytokine, Chemokine, and Growth Factor Profile Characterization of Undifferentiated and Osteoinduced Human Adipose-Derived Stem Cells.Stem Cells Int. 2017;2017:6202783. doi: 10.1155/2017/6202783. Epub 2017 May 10. Stem Cells Int. 2017. PMID: 28572824 Free PMC article.
-
Effects of fish collagen peptides on collagen post-translational modifications and mineralization in an osteoblastic cell culture system.Dent Mater J. 2013;32(1):88-95. doi: 10.4012/dmj.2012-220. Dent Mater J. 2013. PMID: 23370875 Free PMC article.
-
Osteogenic cell differentiation on H-terminated and O-terminated nanocrystalline diamond films.Int J Nanomedicine. 2015 Jan 27;10:869-84. doi: 10.2147/IJN.S73628. eCollection 2015. Int J Nanomedicine. 2015. PMID: 25670900 Free PMC article.
-
Biological Response to Bioinspired Microporous 3D-Printed Scaffolds for Bone Tissue Engineering.Int J Mol Sci. 2022 May 11;23(10):5383. doi: 10.3390/ijms23105383. Int J Mol Sci. 2022. PMID: 35628195 Free PMC article.
-
Surface Free Energy Dominates the Biological Interactions of Postprocessed Additively Manufactured Ti-6Al-4V.ACS Biomater Sci Eng. 2022 Oct 10;8(10):4311-4326. doi: 10.1021/acsbiomaterials.2c00298. Epub 2022 Sep 20. ACS Biomater Sci Eng. 2022. PMID: 36127820 Free PMC article.
References
-
- Eyre DR. Biochemical basis of collagen metabolites as bone turnover markers. In: Raisz L, Rodin G, Bilezikian JP, editors. Principles of Bone Biology. Academic Press; London: 1996. pp. 43–152.
-
- Eyre DR, Wu JJ. Collagen cross-links. Top Curr Chem. 2005;247:207–29.
-
- Kivirikko KI, Myllyla R. Posttranslational enzymes in the biosynthesis of collagen: intracellular enzymes. Methods Enzymol. 1982;82(Pt A):245–304. - PubMed
-
- Hautala T, Byers MG, Eddy RL, Shows TB, Kivirikko KI, Myllyla R. Cloning of human lysyl hydroxylase: complete cDNA-derived amino acid sequence and assignment of the gene (PLOD) to chromosome 1p36.3----p36.2. Genomics. 1992;13:62–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

