Role of nuclear factor kappa B (NF-kappaB) in oxidative stress-induced defective dopamine D1 receptor signaling in the renal proximal tubules of Sprague-Dawley rats
- PMID: 17320758
- PMCID: PMC2696818
- DOI: 10.1016/j.freeradbiomed.2006.11.033
Role of nuclear factor kappa B (NF-kappaB) in oxidative stress-induced defective dopamine D1 receptor signaling in the renal proximal tubules of Sprague-Dawley rats
Abstract
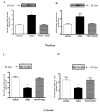
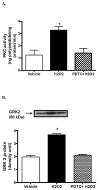
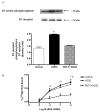
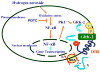
Dopamine promotes sodium excretion, in part, via activation of D1 receptors in renal proximal tubules (PT) and subsequent inhibition of Na, K-ATPase. Recently, we have reported that oxidative stress causes D1 receptor-G-protein uncoupling via mechanisms involving protein kinase C (PKC) and G-protein-coupled receptor kinase 2 (GRK 2) in the primary cultures of renal PT of Sprague-Dawley (SD) rats. There are reports suggesting that redox-sensitive nuclear transcription factor, NF-kappaB, is activated in conditions associated with oxidative stress. This study was designed to identify the role of NF-kappaB in oxidative stress-induced defective renal D1 receptor-G-protein coupling and function. Treatment of the PT with hydrogen peroxide (H(2)O(2), 50 microM/20 min) induced the nuclear translocation of NF-kappaB, increased PKC activity, and triggered the translocation of GRK 2 to the proximal tubular membranes. This was accompanied by hyperphosphorylation of D1 receptors and defective D1 receptor-G-protein coupling. The functional consequence of these changes was decreased D1 receptor activation-mediated inhibition of Na, K-ATPase activity. Interestingly, pretreatment with pyrrolidine dithiocarbamate (PDTC, 25 microM/10 min), an NF-kappaB inhibitor, blocked the H(2)O(2)-induced nuclear translocation of NF-kappaB, increase in PKC activity, and GRK 2 translocation and hyperphosphorylation of D1 receptors in the proximal tubular membranes. Furthermore, PDTC restored D1 receptor G-protein coupling and D1 receptor agonist-mediated inhibition of the Na, K-ATPase activity. Therefore, we suggest that oxidative stress causes nuclear translocation of NF-kappaB in the renal proximal tubules, which contributes to defective D1 receptor-G-protein coupling and function via mechanisms involving PKC, membranous translocation of GRK 2, and subsequent phosphorylation of dopamine D1 receptors.
Figures

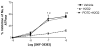
References
-
- Jose PA, Eisner GM, Felder RA. Renal dopamine receptors in health and hypertension. Pharmacol Ther. 1998;80:149–182. - PubMed
-
- Aperia AC. Intrarenal dopamine: a key signal in the interactive regulation of sodium metabolism. Annu Rev Physiol. 2000;62:621–647. - PubMed
-
- Hegde SS, Jadhav AL, Lokhandwala MF. Role of kidney dopamine in the natriuretic response to volume expansion in rats. Hypertension. 1989;13:828–834. - PubMed
-
- Hussain T, Lokhandwala MF. Renal dopamine receptor function in hypertension. Hypertension. 1998;32:187–197. - PubMed
-
- Beheray S, Kansra V, Hussain T, Lokhandwala MF. Diminished natriuretic response to dopamine in old rats is due to an impaired D1-like receptor-signaling pathway. Kidney Int. 2000;58:712–720. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

