ATP-sensitive potassium currents reduce the PGE2-mediated enhancement of excitability in adult rat sensory neurons
- PMID: 17320840
- PMCID: PMC1890028
- DOI: 10.1016/j.brainres.2007.01.103
ATP-sensitive potassium currents reduce the PGE2-mediated enhancement of excitability in adult rat sensory neurons
Abstract
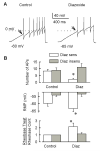
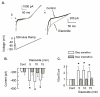
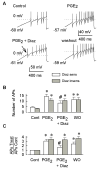
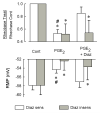
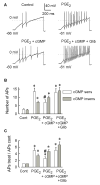
Behavioral studies have shown that the hyperalgesia arising from inflammatory agents, such as prostaglandin E(2) (PGE(2)), can be antagonized by activators of the ATP-sensitive potassium current (K(ATP)). This observation raises questions as to whether this suppression results from a direct action on sensory neurons and what are the cellular mechanisms giving rise to this inhibition. We found that small to medium diameter sensory neurons isolated from the L4-6 DRGs expressed the mRNAs for Kir6.1, Kir6.2, and SUR1. In perforated-patch clamp recordings from acutely dissociated sensory neurons from the young adult rat, exposure to 300 microM diazoxide, a K(ATP) channel agonist, significantly hyperpolarized the resting membrane potential, reduced the number of action potentials evoked by a ramp of depolarizing current, and increased the amplitude of inward K(ATP) currents evoked by the voltage ramp. Similar results were obtained with the protonophore FCCP, which is known to reduce the levels of intracellular ATP and lead to the activation of K(ATP). Only a subpopulation of sensory neurons was sensitive to diazoxide whereas other neurons were unaffected. Treatment with 1 microM PGE(2) significantly enhanced the excitability of these small to medium diameter capsaicin-sensitive sensory neurons; this enhancement was reversed by subsequent exposure to diazoxide in a subpopulation of neurons. Similar to diazoxide, exposure to 8-Br-cyclic GMP antagonized the PGE(2)-induced increase in excitability. The effects of 8-Br-cyclic GMP could be reversed by exposure to glibenclamide, an antagonist of K(ATP) channels. As with diazoxide, only a subpopulation of sensory neurons were affected by 8-Br-cyclic GMP. These results demonstrate that activation of K(ATP) can reverse the sensitization produced by PGE(2) and may be an important means to modulate the enhanced excitability that results from inflammatory or injury conditions.
Figures

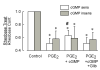
Similar articles
-
ATP-sensitive potassium currents in rat primary afferent neurons: biophysical, pharmacological properties, and alterations by painful nerve injury.Neuroscience. 2009 Aug 18;162(2):431-43. doi: 10.1016/j.neuroscience.2009.04.076. Epub 2009 May 5. Neuroscience. 2009. PMID: 19422886
-
Nitric oxide activates ATP-sensitive potassium channels in mammalian sensory neurons: action by direct S-nitrosylation.Mol Pain. 2009 Mar 14;5:12. doi: 10.1186/1744-8069-5-12. Mol Pain. 2009. PMID: 19284878 Free PMC article.
-
Characterization of K(ATP)-channels in rat basilar and middle cerebral arteries: studies of vasomotor responses and mRNA expression.Eur J Pharmacol. 2005 Oct 31;523(1-3):109-18. doi: 10.1016/j.ejphar.2005.08.028. Epub 2005 Oct 13. Eur J Pharmacol. 2005. PMID: 16226739
-
Towards selective Kir6.2/SUR1 potassium channel openers, medicinal chemistry and therapeutic perspectives.Curr Med Chem. 2006;13(4):361-76. doi: 10.2174/092986706775527947. Curr Med Chem. 2006. PMID: 16475928 Review.
-
Regulation of firing frequency in nociceptive neurons by pro-inflammatory mediators.Exp Brain Res. 2009 Jun;196(1):45-52. doi: 10.1007/s00221-009-1744-2. Epub 2009 Apr 7. Exp Brain Res. 2009. PMID: 19350231 Review.
Cited by
-
Sex differences in the contribution of ATP-sensitive K+ channels in trigeminal ganglia under an acute muscle pain condition.Neuroscience. 2011 Apr 28;180:344-52. doi: 10.1016/j.neuroscience.2011.01.045. Epub 2011 Feb 2. Neuroscience. 2011. PMID: 21296645 Free PMC article.
-
Modulation of SUR1 KATP Channel Subunit Activity in the Peripheral Nervous System Reduces Mechanical Hyperalgesia after Nerve Injury in Mice.Int J Mol Sci. 2019 May 7;20(9):2251. doi: 10.3390/ijms20092251. Int J Mol Sci. 2019. PMID: 31067750 Free PMC article.
-
Loss of SUR1 subtype KATP channels alters antinociception and locomotor activity after opioid administration.Behav Brain Res. 2021 Sep 24;414:113467. doi: 10.1016/j.bbr.2021.113467. Epub 2021 Jul 15. Behav Brain Res. 2021. PMID: 34274374 Free PMC article.
-
Meningeal K ATP channels contribute to behavioral responses in preclinical migraine models.Pain. 2025 Feb 1;166(2):398-407. doi: 10.1097/j.pain.0000000000003385. Epub 2024 Oct 1. Pain. 2025. PMID: 39661370
-
Antinociceptive effects of hydroalcoholic extract from Euterpe oleracea Mart. (Açaí) in a rodent model of acute and neuropathic pain.BMC Complement Altern Med. 2015 Jul 2;15:208. doi: 10.1186/s12906-015-0724-2. BMC Complement Altern Med. 2015. PMID: 26134625 Free PMC article.
References
-
- Aguilar-Bryan L, Clement JP, 4th, Gonzalez G, Kunjilwar K, Babenko A, Bryan J. Toward understanding the assembly and structure of KATP channels. Physiol. Rev. 1998;78:227–245. - PubMed
-
- Alves D, Duarte I. Involvement of ATP-sensitive K+ channels in the peripheral antinociceptive effect induced by dipyrone. Eur. J. Pharmacol. 2002;444:47–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

