Hedgehog signaling regulates the amount of hypaxial muscle development during Xenopus myogenesis
- PMID: 17320852
- PMCID: PMC2080674
- DOI: 10.1016/j.ydbio.2007.01.022
Hedgehog signaling regulates the amount of hypaxial muscle development during Xenopus myogenesis
Abstract
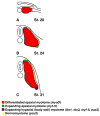
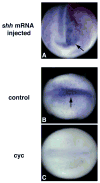
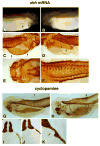
Hedgehog (Hh) signaling is proposed to have different roles on differentiation of hypaxial myoblasts of amniotes. Within the somitic environment, Hh signals restrict hypaxial development and promote epaxial muscle formation. On the other hand, in the limb bud, Hh signaling represses hypaxial myoblast differentiation. This poses the question of whether differences in response to Hh signaling are due to variations in local environment or are intrinsic differences between pre- and post-migratory hypaxial myoblasts. We have approached this question by examining the role of Hh signaling on myoblast development in Xenopus laevis, which, due to its unique mode of hypaxial muscle development, allows us to examine myoblast development in vivo in the absence of the limb environment. Cyclopamine and sonic hedgehog (shh) mRNA overexpression were used to inhibit or activate the Hh pathway, respectively. We find that hypaxial myoblasts respond similarly to Hh manipulations regardless of their location, and that this response is the same for epaxial myoblasts. Overexpression of shh mRNA causes a premature differentiation of the dermomyotome, subsequently inhibiting all further growth of the epaxial and hypaxial myotome. Cyclopamine treatment has the opposite effect, causing an increase in dermomyotome and a shift in myoblast fate from epaxial to hypaxial, eventually leading to an excess of hypaxial body wall muscle. Cyclopamine treatment before stage 20 can rescue the effects of shh overexpression, indicating that early Hh signaling plays an essential role in maintaining the balance between epaxial and hypaxial muscle mass. After stage 20, the premature differentiation of the dermomyotome caused by shh overexpression cannot be rescued by cyclopamine, and no further embryonic muscle growth occurs.
Figures



Similar articles
-
Establishment of the epaxial-hypaxial boundary in the avian myotome.Dev Dyn. 2006 Jul;235(7):1884-94. doi: 10.1002/dvdy.20832. Dev Dyn. 2006. PMID: 16680727
-
The epaxial-hypaxial subdivision of the avian somite.Dev Biol. 2004 Oct 15;274(2):348-69. doi: 10.1016/j.ydbio.2004.07.020. Dev Biol. 2004. PMID: 15385164
-
Hedgehog can drive terminal differentiation of amniote slow skeletal muscle.BMC Dev Biol. 2004 Jul 6;4:9. doi: 10.1186/1471-213X-4-9. BMC Dev Biol. 2004. PMID: 15238161 Free PMC article.
-
Cyclopamine: from cyclops lambs to cancer treatment.J Agric Food Chem. 2014 Jul 30;62(30):7355-62. doi: 10.1021/jf5005622. Epub 2014 Apr 22. J Agric Food Chem. 2014. PMID: 24754790 Review.
-
Skeletal muscle development in the mouse embryo.Histol Histopathol. 2000 Apr;15(2):649-56. doi: 10.14670/HH-15.649. Histol Histopathol. 2000. PMID: 10809386 Review.
Cited by
-
Shh signaling directs dorsal ventral patterning in the regenerating X. tropicalis spinal cord.bioRxiv [Preprint]. 2024 Oct 19:2024.10.18.619160. doi: 10.1101/2024.10.18.619160. bioRxiv. 2024. Update in: Dev Biol. 2025 Apr;520:191-199. doi: 10.1016/j.ydbio.2025.01.015. PMID: 39463962 Free PMC article. Updated. Preprint.
-
Distinct modes of vertebrate hypaxial muscle formation contribute to the teleost body wall musculature.Dev Genes Evol. 2011 Aug;221(3):167-78. doi: 10.1007/s00427-011-0369-1. Epub 2011 Jul 1. Dev Genes Evol. 2011. PMID: 21720828 Free PMC article.
-
Dissecting the pre-placodal transcriptome to reveal presumptive direct targets of Six1 and Eya1 in cranial placodes.Elife. 2016 Aug 31;5:e17666. doi: 10.7554/eLife.17666. Elife. 2016. PMID: 27576864 Free PMC article.
-
A revised model of Xenopus dorsal midline development: differential and separable requirements for Notch and Shh signaling.Dev Biol. 2011 Apr 15;352(2):254-66. doi: 10.1016/j.ydbio.2011.01.021. Epub 2011 Jan 27. Dev Biol. 2011. PMID: 21276789 Free PMC article.
-
Making muscle: Morphogenetic movements and molecular mechanisms of myogenesis in Xenopus laevis.Semin Cell Dev Biol. 2016 Mar;51:80-91. doi: 10.1016/j.semcdb.2016.02.006. Epub 2016 Feb 5. Semin Cell Dev Biol. 2016. PMID: 26853935 Free PMC article. Review.
References
-
- Amthor H, Christ B, Patel K. A molecular mechanism enabling continuous embryonic muscle growth - a balance between proliferation and differentiation. Development. 1999;126:1041–53. - PubMed
-
- Amthor H, Christ B, Weil M, Patel K. The importance of timing differentiation during limb muscle development. Curr Biol. 1998;8:642–52. - PubMed
-
- Bober E, Franz T, Arnold HH, Gruss P, Tremblay P. Pax-3 is required for the development of limb muscles: a possible role for the migration of dermomyotomal muscle progenitor cells. Development. 1994;120:603–12. - PubMed
-
- Borycki AG, Brunk B, Tajbakhsh S, Buckingham M, Chiang C, Emerson CP., Jr Sonic hedgehog controls epaxial muscle determination through Myf5 activation. Development. 1999;126:4053–63. - PubMed
-
- Brand-Saberi B, Christ B. Evolution and development of distinct cell lineages derived from somites. Curr Top Dev Biol. 2000;48:1–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

