The effect of the controlled release of basic fibroblast growth factor from ionic gelatin-based hydrogels on angiogenesis in a murine critical limb ischemic model
- PMID: 17320947
- PMCID: PMC1945227
- DOI: 10.1016/j.biomaterials.2007.01.044
The effect of the controlled release of basic fibroblast growth factor from ionic gelatin-based hydrogels on angiogenesis in a murine critical limb ischemic model
Abstract
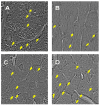
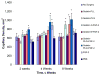
The localized delivery of exogenous, angiogenic growth factors has become a promising alternative treatment of peripheral artery disease (PAD) and critical limb ischemia. In the present study, we describe the development of a novel controlled release vehicle to promote angiogenesis in a murine critical limb ischemic model. Ionic, gelatin-based hydrogels were prepared by the carbodiimide-mediated amidation reaction between the carboxyl groups of gelatin or poly-L-glutamic acid molecules and the amine groups of poly-L-lysine or gelatin molecules, respectively. The degree of swelling of the synthesized hydrogels was assessed as a function of EDC/NHS ratios and the pH of the equilibrating medium, while the release kinetic profile of basic fibroblast growth factor (FGF-2) was evaluated in human fibroblast cultures. The degree of swelling (DS) decreased from 26.5+/-1.7 to 18.5+/-2.4 as the EDC concentration varied from 0.75 to 2.5 mg/ml. Eighty percent of the FGF-2 was released at controlled rates from gelatin-polylysine (gelatin-PLL) and gelatin-polyglutamic acid (gelatin-PLG) hydrogel scaffolds over a period of 28 days. Cell adhesion studies revealed that the negatively charged surface of the gelatin-PLG hydrogels exhibited superior adhesion capabilities in comparison to gelatin-PLL and control gelatin surfaces. Laser Doppler perfusion imaging as well as CD31(+) capillary immunostaining demonstrated that the controlled release of FGF-2 from ionic gelatin-based hydrogels is superior in promoting angiogenesis in comparison to the bolus administration of the growth factor. Over 4 weeks, FGF-2 releasing gelatin-PLG hydrogels exhibited marked reperfusion with a Doppler ratio of 0.889 (+/-0.04) which was 69.3% higher than in the control groups.
Figures




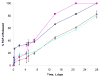
 ), gelatin B (
), gelatin B (
 ), gelatin-PLL (
), gelatin-PLL (
 ), gelatin-PLG (
), gelatin-PLG (
 ) hydrogels was monitored over 28 days (n=3 per time point per group). [gelatin] = 0.1 mg/mL; [PLL or PLG] = 0.01 mg/mL; FGF-2 = 250 ng; [EDC] = 1.3 mg/mL; [NHS] = 0.44 mg/mL.
) hydrogels was monitored over 28 days (n=3 per time point per group). [gelatin] = 0.1 mg/mL; [PLL or PLG] = 0.01 mg/mL; FGF-2 = 250 ng; [EDC] = 1.3 mg/mL; [NHS] = 0.44 mg/mL.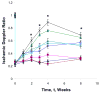
Similar articles
-
Co-delivery of FGF-2 and G-CSF from gelatin-based hydrogels as angiogenic therapy in a murine critical limb ischemic model.Acta Biomater. 2009 Jan;5(1):230-9. doi: 10.1016/j.actbio.2008.07.024. Epub 2008 Aug 3. Acta Biomater. 2009. PMID: 18713669
-
Enhanced angiogenic efficacy through controlled and sustained delivery of FGF-2 and G-CSF from fibrin hydrogels containing ionic-albumin microspheres.J Biomater Sci Polym Ed. 2012;23(1-4):185-206. doi: 10.1163/092050610X546417. Epub 2010 Dec 30. J Biomater Sci Polym Ed. 2012. PMID: 21192837
-
Enhanced angiogenesis by multiple release of platelet-rich plasma contents and basic fibroblast growth factor from gelatin hydrogels.Acta Biomater. 2012 May;8(5):1792-801. doi: 10.1016/j.actbio.2012.01.016. Epub 2012 Jan 21. Acta Biomater. 2012. PMID: 22293581
-
Controlled releases of FGF-2 and paclitaxel from chitosan hydrogels and their subsequent effects on wound repair, angiogenesis, and tumor growth.Curr Drug Deliv. 2006 Oct;3(4):351-8. doi: 10.2174/156720106778559047. Curr Drug Deliv. 2006. PMID: 17076636 Review.
-
[Regenerative medicine with the sustained release system of basic fibroblast growth factor].Nihon Rinsho. 2006 Nov;64(11):2142-7. Nihon Rinsho. 2006. PMID: 17087309 Review. Japanese.
Cited by
-
Liposome-based vascular endothelial growth factor-165 transfection with skeletal myoblast for treatment of ischaemic limb disease.J Cell Mol Med. 2010 Jan;14(1-2):323-36. doi: 10.1111/j.1582-4934.2008.00454.x. Epub 2008 Aug 4. J Cell Mol Med. 2010. PMID: 18681907 Free PMC article.
-
Naturally derived and synthetic scaffolds for skeletal muscle reconstruction.Adv Drug Deliv Rev. 2015 Apr;84:208-21. doi: 10.1016/j.addr.2014.08.011. Epub 2014 Aug 29. Adv Drug Deliv Rev. 2015. PMID: 25174309 Free PMC article. Review.
-
Injectable skeletal muscle matrix hydrogel promotes neovascularization and muscle cell infiltration in a hindlimb ischemia model.Eur Cell Mater. 2012 Jun 5;23:400-12; discussion 412. doi: 10.22203/ecm.v023a31. Eur Cell Mater. 2012. PMID: 22665162 Free PMC article.
-
Skeletal Muscle Tissue Engineering: Biomaterials-Based Strategies for the Treatment of Volumetric Muscle Loss.Bioengineering (Basel). 2020 Jul 31;7(3):85. doi: 10.3390/bioengineering7030085. Bioengineering (Basel). 2020. PMID: 32751847 Free PMC article. Review.
-
Angiogenic therapy for cardiac repair based on protein delivery systems.Heart Fail Rev. 2012 May;17(3):449-73. doi: 10.1007/s10741-011-9285-8. Heart Fail Rev. 2012. PMID: 21979836 Review.
References
-
- Hackam DG, Goodman SG, Anand SS. Management of risk in peripheral artery disease: recent therapeutic advances. Am Heart J. 2005;150:35–40. - PubMed
-
- Aranow WS. Management of peripheral artery disease. Cardiol Rev. 2005;13:61–68. - PubMed
-
- Dashwood MR, Tusi JC. The effect of acute ischemia on ET-1 and its receptors in patients with underlying chronic ischemia of the lower limb. Exp Biol Med. 2006;231:802–805. - PubMed
-
- Parsson H, Holmberg A, Siegbahn A, Berggvist D. Activation of coagulation and fibrinolytic systems in patients with CLI is not normalized after surgical revascularization. Eur J Vasc Surg. 2004;27:186–192. - PubMed
-
- Comerota AJ. Endovascular and surgical revascularization for patients with intermittent claudication. Am J Cardiol. 2001;87(A):34D–43D. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

