Cloning and characterization of a new intestinal inflammation-associated colonic epithelial Ste20-related protein kinase isoform
- PMID: 17321610
- PMCID: PMC1865517
- DOI: 10.1016/j.bbaexp.2007.01.003
Cloning and characterization of a new intestinal inflammation-associated colonic epithelial Ste20-related protein kinase isoform
Abstract
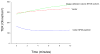
Intestinal epithelial cells respond to inflammatory extracellular stimuli by activating mitogen activated protein kinase (MAPK) signaling, which mediates numerous pathophysiological effects, including intestinal inflammation. Here, we show that a novel isoform of SPS1-related proline alanine-rich kinase (SPAK/STE20) is involved in this inflammatory signaling cascade. We cloned and characterized a SPAK isoform from inflamed colon tissue, and found that this SPAK isoform lacked the characteristic PAPA box and alphaF loop found in SPAK. Based on genomic sequence analysis the lack of PAPA box and alphaF loop in colonic SPAK isoform was the result of specific splicing that affect exon 1 and exon 7 of the SPAK gene. The SPAK isoform was found in inflamed and non-inflamed colon tissues as well as Caco2-BBE cells, but not in other tissues, such as liver, spleen, brain, prostate and kidney. In vitro analyses demonstrated that the SPAK isoform possessed serine/threonine kinase activity, which could be abolished by a substitution of isoleucine for the lysine at position 34 in the ATP-binding site of the catalytic domain. Treatment of Caco2-BBE cells with the pro-inflammatory cytokine, interferon gamma, induced expression of the SPAK isoform. Over-expression of the SPAK isoform in Caco2-BBE cells led to nuclear translocation of an N-terminal fragment of the SPAK isoform, as well as activation of p38 MAP kinase signaling cascades and increased intestinal barrier permeability. These findings collectively suggest that pro-inflammatory cytokine signaling may induce expression of this novel SPAK isoform in intestinal epithelia, triggering the signaling cascades that govern intestinal inflammation.
Figures





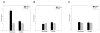

Similar articles
-
Pharmacological targeting of SPAK kinase in disorders of impaired epithelial transport.Expert Opin Ther Targets. 2017 Aug;21(8):795-804. doi: 10.1080/14728222.2017.1351949. Epub 2017 Jul 12. Expert Opin Ther Targets. 2017. PMID: 28679296 Free PMC article. Review.
-
SPAK, a STE20/SPS1-related kinase that activates the p38 pathway.Oncogene. 2000 Aug 31;19(37):4290-7. doi: 10.1038/sj.onc.1203784. Oncogene. 2000. PMID: 10980603
-
Nuclear factor-kappaB is a critical mediator of Ste20-like proline-/alanine-rich kinase regulation in intestinal inflammation.Am J Pathol. 2008 Oct;173(4):1013-28. doi: 10.2353/ajpath.2008.080339. Epub 2008 Sep 11. Am J Pathol. 2008. PMID: 18787102 Free PMC article.
-
Overexpression of Ste20-related proline/alanine-rich kinase exacerbates experimental colitis in mice.J Immunol. 2011 Aug 1;187(3):1496-505. doi: 10.4049/jimmunol.1002910. Epub 2011 Jun 24. J Immunol. 2011. PMID: 21705622 Free PMC article.
-
Ste20-related proline/alanine-rich kinase: a novel regulator of intestinal inflammation.World J Gastroenterol. 2008 Oct 28;14(40):6115-21. doi: 10.3748/wjg.14.6115. World J Gastroenterol. 2008. PMID: 18985800 Free PMC article. Review.
Cited by
-
Evaluation of the Choroid Plexus Epithelium Inflammation TLR4/NF-κB/NKCC1 Signal Pathway Activation in the Development of Hydrocephalus.CNS Neurosci Ther. 2024 Oct;30(10):e70085. doi: 10.1111/cns.70085. CNS Neurosci Ther. 2024. PMID: 39450988 Free PMC article.
-
Pharmacological targeting of SPAK kinase in disorders of impaired epithelial transport.Expert Opin Ther Targets. 2017 Aug;21(8):795-804. doi: 10.1080/14728222.2017.1351949. Epub 2017 Jul 12. Expert Opin Ther Targets. 2017. PMID: 28679296 Free PMC article. Review.
-
The choroid plexus links innate immunity to CSF dysregulation in hydrocephalus.Cell. 2023 Feb 16;186(4):764-785.e21. doi: 10.1016/j.cell.2023.01.017. Cell. 2023. PMID: 36803604 Free PMC article.
-
SPAK Deficiency Attenuates Chemotherapy-Induced Intestinal Mucositis.Front Oncol. 2021 Nov 23;11:733555. doi: 10.3389/fonc.2021.733555. eCollection 2021. Front Oncol. 2021. PMID: 34888232 Free PMC article.
-
Inflammation in acquired hydrocephalus: pathogenic mechanisms and therapeutic targets.Nat Rev Neurol. 2020 May;16(5):285-296. doi: 10.1038/s41582-020-0321-y. Epub 2020 Mar 9. Nat Rev Neurol. 2020. PMID: 32152460 Free PMC article. Review.
References
-
- Waetzig GH, Seegert D, Rosenstiel P, Nikolaus S, Schreiber S. p38 mitogen-activated protein kinase is activated and linked to TNF-alpha signaling in inflammatory bowel disease. J Immunol. 2002;168:5342–5351. - PubMed
-
- Brigitta MN, Brinkman JB, Telliez AR, Schievella LL, Lin AE, Goldfeld AA. Engagement of Tumor Necrosis Factor (TNF) Receptor 1 Leads to ATF-2- and p38 Mitogen-activated Protein Kinase-dependent TNF- Gene Expression. J Biol Chem. 1999;274:30882–30886. - PubMed
-
- Hoffmeyer A, Grosse-Wilde A, Flory E, Neufeld B, Kunz M, Rapp UR, Ludwig S. Different Mitogen-activated Protein Kinase Signaling Pathways Cooperate to Regulate Tumor Necrosis Factor α Gene Expression in T Lymphocytes. J Biol Chem. 1999;274:4319–4327. - PubMed
-
- Hampe J, Shaw SH, Saiz R, Leysens N, Lantermann A, Mascheretti S, Lynch NJ, MacPherson AJ, Bridger S, van Deventer S, Stokkers P, Morin P, Mirza MM, Forbes A, Lennard-Jones JE, Mathew CG, Curran ME, Schreiber S. Linkage of inflammatory bowel disease to human chromosome 6p. Am J Hum Genet. 1999;65:647–1655. - PMC - PubMed
-
- Hugot JP, Laurent-Puig P, Gower-Rousseau C, Olson JM, Lee JC, Beaugerie L, Naom I, Dupas JL, Van Gossum A, Orholm M, Bonaiti-Pellie C, Weissenbach J, Mathew CG, Lennard-Jones JE, Cortot A, Colombel JF, Thomas G. Mapping of a susceptibility locus for Crohn’s disease on chromosome 16. Nature. 1996;379:772–773. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

