Biofilms formed by nontypeable Haemophilus influenzae in vivo contain both double-stranded DNA and type IV pilin protein
- PMID: 17322318
- PMCID: PMC1913342
- DOI: 10.1128/JB.01935-06
Biofilms formed by nontypeable Haemophilus influenzae in vivo contain both double-stranded DNA and type IV pilin protein
Abstract
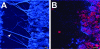
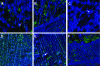
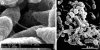
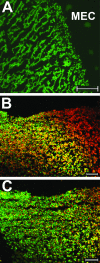
Nontypeable Haemophilus influenzae (NTHI) strains are members of the normal human nasopharyngeal flora, as well as frequent opportunistic pathogens of both the upper and lower respiratory tracts. Recently, it has been shown that NTHI can form biofilms both in vitro and in vivo. NTHI strains within in vitro-formed biofilms differentially express both epitopes of lipooligosaccharide (LOS) and the outer membrane proteins P2, P5, and P6, whereas those generated either in a 96-well plate assay in vitro or in a mammalian host have been shown to incorporate a specific glycoform of sialylated LOS within the biofilm matrix. While DNA has been identified as a key component of the biofilm matrix formed in vitro by several bacterial pathogens, here we demonstrate for the first time that in addition to sialylated LOS, the biofilm formed by NTHI in vivo contains both type IV pilin protein and a significant amount of double-stranded DNA. The DNA appeared to be arranged in a dense interlaced meshwork of fine strands as well as in individual thicker "ropes" that span water channels, suggesting that DNA could be imparting structural stability to the biofilm produced by NTHI in vivo. The presence of type IV pilin protein both appearing as small aggregates within the biofilm matrix and tracking along DNA strands supports our observations which showed that type IV pili are expressed by NTHI during experimental otitis media when these bacteria form a biofilm in the middle ear space.
Figures




References
-
- Aas, F. E., M. Wolfgang, S. Frye, S. Dunham, C. Lovold, and M. Koomey. 2002. Competence for natural transformation in Neisseria gonorrhoeae: components of DNA binding and uptake linked to type IV pilus expression. Mol. Microbiol. 46:749-760. - PubMed
-
- Allesen-Holm, M., K. B. Barken, L. Yang, M. Klausen, J. S. Webb, S. Kjelleberg, S. Molin, M. Givskov, and T. Tolker-Nielsen. 2006. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol. Microbiol. 59:1114-1128. - PubMed
-
- Bakaletz, L. O. 2002. Otitis media, p. 259-298. In K. A. Brogden and J. M. Guthmiller (ed.), Polymicrobial diseases. ASM Press, Washington, DC. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

