Generation by a widely applicable approach of a hybrid dioxygenase showing improved oxidation of polychlorobiphenyls
- PMID: 17322323
- PMCID: PMC1855580
- DOI: 10.1128/AEM.02523-06
Generation by a widely applicable approach of a hybrid dioxygenase showing improved oxidation of polychlorobiphenyls
Abstract
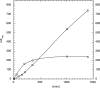
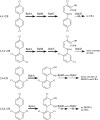
Recently, a sequence-based approach has been developed for the fast isolation and characterization of class II aryl-hydroxylating dioxygenase activities (S. Kahl and B. Hofer, Microbiology 149:1475-1481, 2003). It comprises the PCR amplification of segments of alpha subunit genes of unknown sequence that encode the catalytic center and their fusion with sequences of the bphA gene cluster of Burkholderia xenovorans LB400. One of the resulting chimeric enzymes, harboring the core segment of a dioxygenase from Pseudomonas sp. strain B4-Magdeburg, has now been characterized with respect to the oxidation of chlorobiphenyls (CBs). Its substrate and product specificities differed favorably from those of the parental dioxygenase of strain LB400. The hybrid possessed a higher regiospecificity and yielded less unproductive dioxygenations at meta and para carbons. It attacked ortho-, meta-, and para-chlorinated rings with comparable efficiencies. It gave significantly higher yields in ortho,meta-dioxygenation of recalcitrant congeners containing a doubly ortho-chlorinated ring. While the parental enzyme yielded mainly unproductive meta, para dioxygenation of 2,5,4'-CB, the hybrid predominantly converted this congener into an ortho,meta-dioxygenated product. The subsequent enzymes of the LB400 catabolic pathway were able to transform most of the metabolites formed by the novel dioxygenase, indicating that the substrate ranges of these biocatalysts are not adapted to that of their initial pathway enzyme. Some of the catabolites, however, were identified as problematic for further degradation. Our results demonstrate that the outlined approach can successfully be applied to obtain novel dioxygenase specificities that favorably complement or supplement known ones.
Figures



Similar articles
-
An aryl dioxygenase shows remarkable double dioxygenation capacity for diverse bis-aryl compounds, provided they are carbocyclic.Appl Microbiol Biotechnol. 2016 Sep;100(18):8053-61. doi: 10.1007/s00253-016-7570-0. Epub 2016 May 5. Appl Microbiol Biotechnol. 2016. PMID: 27147529
-
Family shuffling of soil DNA to change the regiospecificity of Burkholderia xenovorans LB400 biphenyl dioxygenase.J Bacteriol. 2007 Feb;189(3):779-88. doi: 10.1128/JB.01267-06. Epub 2006 Dec 1. J Bacteriol. 2007. PMID: 17142386 Free PMC article.
-
Heterologous expression of biphenyl dioxygenase-encoding genes from a gram-positive broad-spectrum polychlorinated biphenyl degrader and characterization of chlorobiphenyl oxidation by the gene products.J Bacteriol. 1997 Mar;179(6):1924-30. doi: 10.1128/jb.179.6.1924-1930.1997. J Bacteriol. 1997. PMID: 9068637 Free PMC article.
-
Genetic construction of PCB degraders.Biodegradation. 1994 Dec;5(3-4):359-77. doi: 10.1007/BF00696470. Biodegradation. 1994. PMID: 7765843 Review.
-
α-Dioxygenases (α-DOXs): Promising Biocatalysts for the Environmentally Friendly Production of Aroma Compounds.Chembiochem. 2022 Jun 20;23(12):e202100693. doi: 10.1002/cbic.202100693. Epub 2022 Feb 15. Chembiochem. 2022. PMID: 35107200 Free PMC article. Review.
Cited by
-
Characterization of biphenyl dioxygenase sequences and activities encoded by the metagenomes of highly polychlorobiphenyl-contaminated soils.Appl Environ Microbiol. 2012 Apr;78(8):2706-15. doi: 10.1128/AEM.07381-11. Epub 2012 Feb 10. Appl Environ Microbiol. 2012. PMID: 22327590 Free PMC article.
References
-
- Ahmed, M., and D. D. Focht. 1973. Degradation of polychlorinated biphenyls by two species of Achromobacter. Can. J. Microbiol. 19:47-52. - PubMed
-
- Barriault, D., C. Simard, H. Chatel, and M. Sylvestre. 2001. Characterization of hybrid biphenyl dioxygenases obtained by recombining Burkholderia sp. strain LB400 bphA with the homologous gene of Comamonas testosteroni B-356. Can. J. Microbiol. 47:1025-1032. - PubMed
-
- Barriault, D., F. Lepine, M. Mohammadi, S. Milot, N. Leberre, and M. Sylvestre. 2004. Revisiting the regiospecificity of Burkholderia xenovorans LB400 biphenyl dioxygenase toward 2,2′-dichlorobiphenyl and 2,3,2′,3′-tetrachlorobiphenyl. J. Biol. Chem. 279:47489-47496. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

