Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice
- PMID: 17322368
- PMCID: PMC1864893
- DOI: 10.2353/ajpath.2007.060547
Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice
Abstract
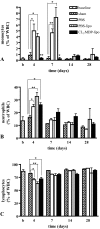
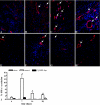
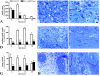
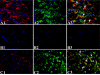
Macrophages have been suggested to be beneficial for myocardial wound healing. We investigated the role of macrophages in myocardial wound healing by inhibition of macrophage infiltration after myocardial injury. We used a murine cryoinjury model to induce left ventricular damage. Infiltrating macrophages were depleted during the 1st week after cryoinjury by serial intravenous injections of clodronate-containing liposomes. After injury, the presence of macrophages, which secreted high levels of transforming growth factor-beta and vascular endothelial growth factor-A, led to rapid removal of cell debris and replacement by granulation tissue containing inflammatory cells and blood vessels, followed by myofibroblast infiltration and collagen deposition. In macrophage-depleted hearts, nonresorbed cell debris was still observed 4 weeks after injury. Secretion of transforming growth factor-beta and vascular endothelial growth factor-A as well as neovascularization, myofibroblast infiltration, and collagen deposition decreased. Moreover, macrophage depletion resulted in a high mortality rate accompanied by increased left ventricular dilatation and wall thinning. In conclusion, infiltrating macrophage depletion markedly impairs wound healing and increases remodeling and mortality after myocardial injury, identifying the macrophage as a key player in myocardial wound healing. Based on these findings, we propose that increasing macrophage numbers early after myocardial infarction could be a clinically relevant option to promote myocardial wound healing and subsequently to reduce remodeling and heart failure.
Figures






Similar articles
-
Effect of granulocyte-macrophage colony-stimulating factor inducer on left ventricular remodeling after acute myocardial infarction.J Am Coll Cardiol. 2004 Oct 6;44(7):1510-20. doi: 10.1016/j.jacc.2004.05.083. J Am Coll Cardiol. 2004. PMID: 15464336
-
Regulatory role of dendritic cells in postinfarction healing and left ventricular remodeling.Circulation. 2012 Mar 13;125(10):1234-45. doi: 10.1161/CIRCULATIONAHA.111.052126. Epub 2012 Feb 3. Circulation. 2012. PMID: 22308302
-
Ex vivo activated human macrophages improve healing, remodeling, and function of the infarcted heart.Circulation. 2006 Jul 4;114(1 Suppl):I94-100. doi: 10.1161/CIRCULATIONAHA.105.000331. Circulation. 2006. PMID: 16820652
-
Role of lymphocytes in myocardial injury, healing, and remodeling after myocardial infarction.Circ Res. 2015 Jan 16;116(2):354-67. doi: 10.1161/CIRCRESAHA.116.304072. Circ Res. 2015. PMID: 25593279 Review.
-
Myocardial fibrosis seen through the lenses of T-cell biology.J Mol Cell Cardiol. 2016 Mar;92:41-5. doi: 10.1016/j.yjmcc.2016.01.018. Epub 2016 Jan 22. J Mol Cell Cardiol. 2016. PMID: 26804387 Review.
Cited by
-
Acceleration of wound healing by α-gal nanoparticles interacting with the natural anti-Gal antibody.J Immunol Res. 2015;2015:589648. doi: 10.1155/2015/589648. Epub 2015 Apr 1. J Immunol Res. 2015. PMID: 25922849 Free PMC article. Review.
-
Can heart function lost to disease be regenerated by therapeutic targeting of cardiac scar tissue?Semin Cell Dev Biol. 2016 Oct;58:41-54. doi: 10.1016/j.semcdb.2016.05.020. Epub 2016 May 24. Semin Cell Dev Biol. 2016. PMID: 27234380 Free PMC article. Review.
-
Tissue microenvironments define and get reinforced by macrophage phenotypes in homeostasis or during inflammation, repair and fibrosis.J Innate Immun. 2012;4(5-6):463-77. doi: 10.1159/000336717. Epub 2012 Apr 11. J Innate Immun. 2012. PMID: 22507825 Free PMC article. Review.
-
Role of innate and adaptive immune mechanisms in cardiac injury and repair.Nat Rev Immunol. 2015 Feb;15(2):117-29. doi: 10.1038/nri3800. Nat Rev Immunol. 2015. PMID: 25614321 Free PMC article. Review.
-
Reversal of aging-associated increase in myelopoiesis and expression of alarmins by angiotensin-(1-7).Sci Rep. 2023 Feb 13;13(1):2543. doi: 10.1038/s41598-023-29853-w. Sci Rep. 2023. PMID: 36782016 Free PMC article.
References
-
- Diez-Roux G, Lang RA. Macrophages induce apoptosis in normal cells in vivo. Development. 1997;124:3633–3638. - PubMed
-
- Leibovich SJ, Wiseman DM. Macrophages, wound repair and angiogenesis. Prog Clin Biol Res. 1988;266:131–145. - PubMed
-
- Mustoe TA, Pierce GF, Thomason A, Gramates P, Sporn MB, Deuel TF. Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science. 1987;237:1333–1336. - PubMed
-
- Leibovich SJ, Danon D. Promotion of wound repair in mice by application of glucan. J Reticuloendothel Soc. 1980;27:1–11. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

