Essential roles of the CC chemokine ligand 3-CC chemokine receptor 5 axis in bleomycin-induced pulmonary fibrosis through regulation of macrophage and fibrocyte infiltration
- PMID: 17322370
- PMCID: PMC1864886
- DOI: 10.2353/ajpath.2007.051213
Essential roles of the CC chemokine ligand 3-CC chemokine receptor 5 axis in bleomycin-induced pulmonary fibrosis through regulation of macrophage and fibrocyte infiltration
Abstract
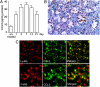
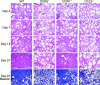
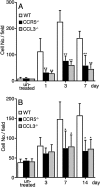
We investigated the pathogenic roles of CC chemokine ligand (CCL)3 and its receptors, CC chemokine receptor (CCR)1 and CCR5, in bleomycin (BLM)-induced pulmonary fibrosis (PF). An intratracheal injection of BLM into wild-type (WT) mice caused a massive infiltration of granulocytes and macrophages, followed by the development of diffuse PF with fibrocyte accumulation. Intrapulmonary CCL3 expression was enhanced rapidly and remained at elevated levels until PF developed. Moreover, CCL3 protein was detected mainly in infiltrating granulocytes and macrophages, whereas transforming growth factor-beta1 protein was detected in macrophages and myofibroblasts. Compared with WT mice, collagen accumulation was reduced in CCL3(-/-) and CCR5(-/-) but not CCR1(-/-) mice. Moreover, the BLM-induced increases in intrapulmonary macrophage and fibrocyte numbers were attenuated in CCL3(-/-) and CCR5(-/-) but not CCR1(-/-) mice, although BLM increased bone marrow (BM) fibrocyte number to a similar extent in these strains. BM transplantation from CCR5(-/-) to WT, but not that from WT to CCR5(-/-) mice, recapitulated the phenotypes in CCR5(-/-) mice. Furthermore, CCR5(+/-) mice exhibited a significant reduction in BLM-induced fibrotic changes. These results demonstrated that locally produced CCL3 was involved in BLM-induced recruitment of BM-derived macrophages and fibrocytes, main producers of transforming growth factor-beta1, and subsequent development of PF by interacting mainly with CCR5.
Figures




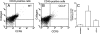

References
-
- Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med. 2001;345:517–525. - PubMed
-
- American Thoracic Society Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med. 2000;161:646–664. - PubMed
-
- Chabner BA, Ryan DP, Paz-Ares L, Garcia-Carbonero R, Calabresi P. Antineoplastic agents. Hardman JG, Limbird LE, editors. New York: McGraw-Hill,; The Pharmacological Basis of Therapeutics, (ed 10) 2003:pp 1429–1430.
-
- Bowden DH. Unraveling pulmonary fibrosis: the bleomycin model. Lab Invest. 1984;50:487–488. - PubMed
-
- Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160:419–425. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

