Aryl hydrocarbon receptor-deficient mice develop heightened inflammatory responses to cigarette smoke and endotoxin associated with rapid loss of the nuclear factor-kappaB component RelB
- PMID: 17322371
- PMCID: PMC1864867
- DOI: 10.2353/ajpath.2007.060391
Aryl hydrocarbon receptor-deficient mice develop heightened inflammatory responses to cigarette smoke and endotoxin associated with rapid loss of the nuclear factor-kappaB component RelB
Abstract
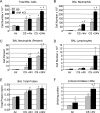
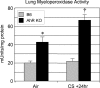
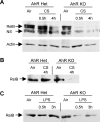
The transcription factor aryl hydrocarbon receptor (AhR) plays an important role in the response to environmental pollutants. However, its role in normal physiology is unclear. To investigate the role of AhR in acute lung inflammation, control and AhR knockout (KO) mice were exposed to inhaled cigarette smoke or bacterial endotoxin. Smoke-induced lung inflammation was twofold to threefold more severe in AhR KO mice than controls. Intriguingly, levels of tumor necrosis factor-alpha and interleukin-6 in the bronchoalveolar lavage of air-exposed KO mice were equal to the levels seen in smoke-exposed controls, suggesting that AhR-deficient mice are inflammation prone. AhR KO mice challenged with inhaled endotoxin, which does not contain AhR ligands, also developed greater lung neutrophilia than controls, and bronchoalveolar lavage cells from AhR KO mice produced elevated levels of tumor necrosis factor-alpha and interleukin-6 when treated with endotoxin in vitro. Nuclear factor-kappaB DNA-binding activity was elevated in smoke-exposed AhR KO mice compared with controls and was associated with a rapid loss of RelB only in the KO mice. We propose that AhR is a previously unrecognized regulator of inflammation that interacts with nuclear factor-kappaB so that in the absence of AhR RelB is prematurely degraded, resulting in heightened inflammatory responses to multiple proinflam-matory stimuli.
Figures





References
-
- Di Stefano A, Caramori G, Ricciardolo FL, Capelli A, Adcock IM, Donner CF. Cellular and molecular mechanisms in chronic obstructive pulmonary disease: an overview. Clin Exp Allergy. 2004;34:1156–1167. - PubMed
-
- Wright JG, Christman JW. The role of nuclear factor kappa B in the pathogenesis of pulmonary diseases: implications for therapy. Am J Respir Med. 2003;2:211–219. - PubMed
-
- Churg A, Zay K, Shay S, Xie C, Shapiro SD, Hendricks R, Wright JL. Acute cigarette smoke-induced connective tissue breakdown requires both neutrophils and macrophage metalloelastase in mice. Am J Respir Cell Mol Biol. 2002;27:368–374. - PubMed
-
- Miller LM, Foster WM, Dambach DM, Doebler D, McKinnon M, Killar L, Longphre M. A murine model of cigarette smoke-induced pulmonary inflammation using intranasally administered smoke-conditioned medium. Exp Lung Res. 2002;28:435–455. - PubMed
-
- Spurzem JR, Rennard SI. Pathogenesis of COPD. Semin Respir Crit Care Med. 2005;26:142–153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS054578/NS/NINDS NIH HHS/United States
- HL075432/HL/NHLBI NIH HHS/United States
- EY017123/EY/NEI NIH HHS/United States
- DE011390/DE/NIDCR NIH HHS/United States
- R01 EY017123/EY/NEI NIH HHS/United States
- P30 ES001247/ES/NIEHS NIH HHS/United States
- P30ES01247/ES/NIEHS NIH HHS/United States
- R01 DE011390/DE/NIDCR NIH HHS/United States
- NS054578/NS/NINDS NIH HHS/United States
- R01 ES007026/ES/NIEHS NIH HHS/United States
- R01 ES009430/ES/NIEHS NIH HHS/United States
- R01 HL078603/HL/NHLBI NIH HHS/United States
- ES-07026/ES/NIEHS NIH HHS/United States
- ES09430/ES/NIEHS NIH HHS/United States
- HL078603/HL/NHLBI NIH HHS/United States
- K08 HL004492/HL/NHLBI NIH HHS/United States
- R01 HL075432/HL/NHLBI NIH HHS/United States
- K08HL04492/HL/NHLBI NIH HHS/United States
- T32 ES007026/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

