Role of SVP in the control of flowering time by ambient temperature in Arabidopsis
- PMID: 17322399
- PMCID: PMC1804328
- DOI: 10.1101/gad.1518407
Role of SVP in the control of flowering time by ambient temperature in Arabidopsis
Abstract
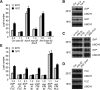
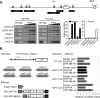
Plants must perceive and rapidly respond to changes in ambient temperature for their successful reproduction. Here we demonstrate that Arabidopsis SHORT VEGETATIVE PHASE (SVP) plays an important role in the response of plants to ambient temperature changes. The loss of SVP function elicited insensitivity to ambient temperature changes. SVP mediates the temperature-dependent functions of FCA and FVE within the thermosensory pathway. SVP controls flowering time by negatively regulating the expression of a floral integrator, FLOWERING LOCUS T (FT), via direct binding to the CArG motifs in the FT sequence. We propose that this is one of the molecular mechanisms that modulate flowering time under fluctuating temperature conditions.
Figures


References
-
- Abe M., Kobayashi Y., Yamamoto S., Daimon Y., Yamaguchi A., Ikeda Y., Ichinoki H., Notaguchi M., Goto K., Araki T., Kobayashi Y., Yamamoto S., Daimon Y., Yamaguchi A., Ikeda Y., Ichinoki H., Notaguchi M., Goto K., Araki T., Yamamoto S., Daimon Y., Yamaguchi A., Ikeda Y., Ichinoki H., Notaguchi M., Goto K., Araki T., Daimon Y., Yamaguchi A., Ikeda Y., Ichinoki H., Notaguchi M., Goto K., Araki T., Yamaguchi A., Ikeda Y., Ichinoki H., Notaguchi M., Goto K., Araki T., Ikeda Y., Ichinoki H., Notaguchi M., Goto K., Araki T., Ichinoki H., Notaguchi M., Goto K., Araki T., Notaguchi M., Goto K., Araki T., Goto K., Araki T., Araki T. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science. 2005;309:1052–1056. - PubMed
-
- Alonso J.M., Stepanova A.N., Leisse T.J., Kim C.J., Chen H., Shinn P., Stevenson D.K., Zimmerman J., Barajas P., Cheuk R., Stepanova A.N., Leisse T.J., Kim C.J., Chen H., Shinn P., Stevenson D.K., Zimmerman J., Barajas P., Cheuk R., Leisse T.J., Kim C.J., Chen H., Shinn P., Stevenson D.K., Zimmerman J., Barajas P., Cheuk R., Kim C.J., Chen H., Shinn P., Stevenson D.K., Zimmerman J., Barajas P., Cheuk R., Chen H., Shinn P., Stevenson D.K., Zimmerman J., Barajas P., Cheuk R., Shinn P., Stevenson D.K., Zimmerman J., Barajas P., Cheuk R., Stevenson D.K., Zimmerman J., Barajas P., Cheuk R., Zimmerman J., Barajas P., Cheuk R., Barajas P., Cheuk R., Cheuk R., et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301:653–657. - PubMed
-
- Atkin O.K., Tjoelker M.G., Tjoelker M.G. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci. 2003;8:343–351. - PubMed
-
- Blázquez M.A., Ahn J.H., Weigel D., Ahn J.H., Weigel D., Weigel D. A thermosensory pathway controlling flowering time in Arabidopsis thaliana. Nat. Genet. 2003;33:168–171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
