Functional interactions between the Moses corepressor and DHR78 nuclear receptor regulate growth in Drosophila
- PMID: 17322404
- PMCID: PMC1804333
- DOI: 10.1101/gad.1519007
Functional interactions between the Moses corepressor and DHR78 nuclear receptor regulate growth in Drosophila
Abstract
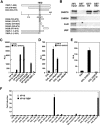
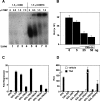
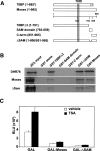
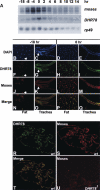
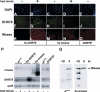
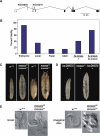
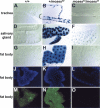
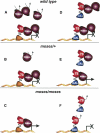
Expression of the Drosophila orphan nuclear receptor DHR78 is regulated by the steroid hormone ecdysone and is required for growth and viability during larval stages. In contrast to our understanding of its biological functions, however, relatively little is known about how DHR78 acts as a transcription factor. Here we show that DHR78 is an obligate partner for Moses (Middleman of seventy-eight signaling), a SAM (sterile alpha motif) domain-containing cofactor that requires DHR78 for its stability. Unlike other nuclear receptor cofactors, Moses has no obvious interaction domains and displays a unique binding specificity for DHR78. Moses acts as a corepressor, inhibiting DHR78 transcriptional activity independently of histone deacetylation. Consistent with their close association, DHR78 and Moses proteins are coexpressed during development and colocalize to specific genomic targets in chromatin. Moses mutants progress normally through early larval stages, like DHR78 mutants, but display an opposite overgrowth phenotype, with hypertrophy of adult tissues. Genetic interactions between DHR78 and moses result in a similar phenotype, suggesting that the relative dose of Moses and DHR78 regulates growth and prevents cancer. The tight functional association between DHR78 and Moses provides a new paradigm for understanding the molecular mechanisms by which cofactors modulate nuclear receptor signaling pathways.
Figures
Similar articles
-
The DHR78 nuclear receptor is required for ecdysteroid signaling during the onset of Drosophila metamorphosis.Cell. 1998 May 15;93(4):543-55. doi: 10.1016/s0092-8674(00)81184-8. Cell. 1998. PMID: 9604930
-
Essential roles for the Dhr78 orphan nuclear receptor during molting of the Drosophila tracheal system.Insect Biochem Mol Biol. 2003 Dec;33(12):1201-9. doi: 10.1016/j.ibmb.2003.06.011. Insect Biochem Mol Biol. 2003. PMID: 14599492
-
Adult functions for the Drosophila DHR78 nuclear receptor.Dev Dyn. 2018 Feb;247(2):315-322. doi: 10.1002/dvdy.24608. Epub 2017 Dec 12. Dev Dyn. 2018. PMID: 29171103 Free PMC article.
-
A tale of tailless.Dev Neurosci. 2011;33(1):1-13. doi: 10.1159/000321585. Epub 2010 Dec 2. Dev Neurosci. 2011. PMID: 21124006 Free PMC article. Review.
-
N-CoR-HDAC corepressor complexes: roles in transcriptional regulation by nuclear hormone receptors.Curr Top Microbiol Immunol. 2003;274:237-68. doi: 10.1007/978-3-642-55747-7_9. Curr Top Microbiol Immunol. 2003. PMID: 12596910 Review.
Cited by
-
HIF- and non-HIF-regulated hypoxic responses require the estrogen-related receptor in Drosophila melanogaster.PLoS Genet. 2013;9(1):e1003230. doi: 10.1371/journal.pgen.1003230. Epub 2013 Jan 31. PLoS Genet. 2013. PMID: 23382692 Free PMC article.
-
Elucidating the 'Jekyll and Hyde' nature of PXR: the case for discovering antagonists or allosteric antagonists.Pharm Res. 2009 Aug;26(8):1807-15. doi: 10.1007/s11095-009-9901-7. Epub 2009 May 5. Pharm Res. 2009. PMID: 19415465 Free PMC article. Review.
-
CONSERVED AND EXAPTED FUNCTIONS OF NUCLEAR RECEPTORS IN ANIMAL DEVELOPMENT.Nucl Receptor Res. 2017;4:101305. doi: 10.11131/2017/101305. Nucl Receptor Res. 2017. PMID: 29333434 Free PMC article.
-
Bradysia (Sciara) coprophila larvae up-regulate DNA repair pathways and down-regulate developmental regulators in response to ionizing radiation.Genetics. 2024 Mar 6;226(3):iyad208. doi: 10.1093/genetics/iyad208. Genetics. 2024. PMID: 38066617 Free PMC article.
-
Samd7 is a cell type-specific PRC1 component essential for establishing retinal rod photoreceptor identity.Proc Natl Acad Sci U S A. 2017 Sep 26;114(39):E8264-E8273. doi: 10.1073/pnas.1707021114. Epub 2017 Sep 12. Proc Natl Acad Sci U S A. 2017. PMID: 28900001 Free PMC article.
References
-
- Andres A.J., Thummel C.S., Thummel C.S. Methods for quantitative analysis of transcription in larvae and prepupae. In: Goldstein L.S.B., Fyrberg E.A., Fyrberg E.A., editors. Drosophila melanogaster: Practical uses in cell and molecular biology. Academic Press; New York: 1994. pp. 565–573. - PubMed
-
- Andrew D.J., Scott M.P., Scott M.P. Immunological method for mapping protein distributions on polytene chromosomes. In: Goldstein L.S.B., Fyrberg E.A., Fyrberg E.A., editors. Drosophila melanogaster: Practical uses in cell and molecular biology. Academic Press; New York: 1994. pp. 353–370. - PubMed
-
- Baker K.D. “Characterization of a second receptor for ecdysteroids in Drosophila and the isolation of a novel orphan receptor-binding protein.” Ph.D. thesis, University of Texas Southwestern Medical Center, Dallas, TX. 2002.
-
- Baker K.D., Warren J.T., Thummel C.S., Gilbert L.I., Mangelsdorf D.J., Warren J.T., Thummel C.S., Gilbert L.I., Mangelsdorf D.J., Thummel C.S., Gilbert L.I., Mangelsdorf D.J., Gilbert L.I., Mangelsdorf D.J., Mangelsdorf D.J. Transcriptional activation of the Drosophila ecdysone receptor by insect and plant ecdysteroids. Insect. Biochem. Mol. Biol. 2000;30:1037–1043. - PubMed
-
- Baker K.D., Shewchuk L.M., Kozlova T., Makishima M., Hassell A., Wisely B., Caravella J.A., Lambert M.H., Reinking J.L., Krause H., Shewchuk L.M., Kozlova T., Makishima M., Hassell A., Wisely B., Caravella J.A., Lambert M.H., Reinking J.L., Krause H., Kozlova T., Makishima M., Hassell A., Wisely B., Caravella J.A., Lambert M.H., Reinking J.L., Krause H., Makishima M., Hassell A., Wisely B., Caravella J.A., Lambert M.H., Reinking J.L., Krause H., Hassell A., Wisely B., Caravella J.A., Lambert M.H., Reinking J.L., Krause H., Wisely B., Caravella J.A., Lambert M.H., Reinking J.L., Krause H., Caravella J.A., Lambert M.H., Reinking J.L., Krause H., Lambert M.H., Reinking J.L., Krause H., Reinking J.L., Krause H., Krause H., et al. The Drosophila orphan nuclear receptor DHR38 mediates an atypical ecdysteroid signaling pathway. Cell. 2003;113:731–742. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
