Arabidopsis irregular xylem8 and irregular xylem9: implications for the complexity of glucuronoxylan biosynthesis
- PMID: 17322407
- PMCID: PMC1867335
- DOI: 10.1105/tpc.106.049320
Arabidopsis irregular xylem8 and irregular xylem9: implications for the complexity of glucuronoxylan biosynthesis
Abstract
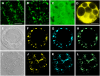
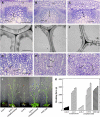
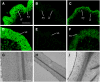
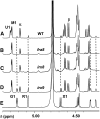
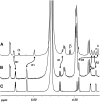
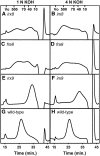
Mutations of Arabidopsis thaliana IRREGULAR XYLEM8 (IRX8) and IRX9 were previously shown to cause a collapsed xylem phenotype and decreases in xylose and cellulose in cell walls. In this study, we characterized IRX8 and IRX9 and performed chemical and structural analyses of glucuronoxylan (GX) from irx8 and irx9 plants. IRX8 and IRX9 are expressed specifically in cells undergoing secondary wall thickening, and their encoded proteins are targeted to the Golgi, where GX is synthesized. 1H-NMR spectroscopy showed that the reducing end of Arabidopsis GX contains the glycosyl sequence 4-beta-D-Xylp-(1-->4)-beta-D-Xylp-(1-->3)-alpha-L-Rhap-(1-->2)-alpha-D-GalpA-(1-->4)-D-Xylp, which was previously identified in birch (Betula verrucosa) and spruce (Picea abies) GX. This indicates that the reducing end structure of GXs is evolutionarily conserved in woody and herbaceous plants. This sequence is more abundant in irx9 GX than in the wild type, whereas irx8 and fragile fiber8 (fra8) plants are nearly devoid of it. The number of GX chains increased and the GX chain length decreased in irx9 plants. Conversely, the number of GX chains decreased and the chain length heterodispersity increased in irx8 and fra8 plants. Our results suggest that IRX9 is required for normal GX elongation and indicate roles for IRX8 and FRA8 in the synthesis of the glycosyl sequence at the GX reducing end.
Figures


References
-
- Andersson, S.-I., Samuelson, O., Ishihara, M., and Shimizu, K. (1983). Structure of the reducing end-groups in spruce xylan. Carbohydr. Res. 111 283–288.
-
- Baydoun, E.A.-H., and Brett, C.T. (1997). Distribution of xylosyltransferases and glucuronyltransferase within the Golgi apparatus in etiolated pea (Pisum sativum L) epicotyls. J. Exp. Bot. 48 1209–1214.
NOTE ADDED IN PROOF
-
- After acceptance of this manuscript, Persson et al. (2007) published an analysis showing altered secondary wall morphology and reduced xylan and homogalacturonan content in the cell walls of irx8 plants.
-
- Persson, S., Caffall, K.H., Freshour, G., Hilley, M.T., Bauer, S., Poindexter, P., Hahn, M.G., Mohnen, D., and Somerville, C. (2007). The Arabidopsis irregular xylem8 mutant is deficient in glucuronoxylan and homogalacturonan, which are essential for secondary cell wall integrity. Plant Cell 19 237–255. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

